Scientists have developed a groundbreaking electrochemical technique that can recover fingerprints from fired brass ammunition casings, something long considered impossible due to the heat and friction of gunfire.



Researchers at University of Limerick (UL) have developed a battery that could reshape the future of electric vehicles and portable electronics. Their breakthrough in energy storage technology has seen the development of the world’s first full-cell dual-cation battery.
This innovative system combines lithium and sodium ions to significantly enhance both battery capacity and stability, marking a new frontier in sustainable energy research.
The work, published in Nano Energy, was led by Hugh Geaney, Associate Professor of Chemistry at UL’s Department of Chemical Sciences and Principal Investigator at UL’s Bernal Institute, and Government of Ireland postdoctoral fellow, Dr. Syed Abdul Ahad, his colleague at the Department and the Bernal Institute.
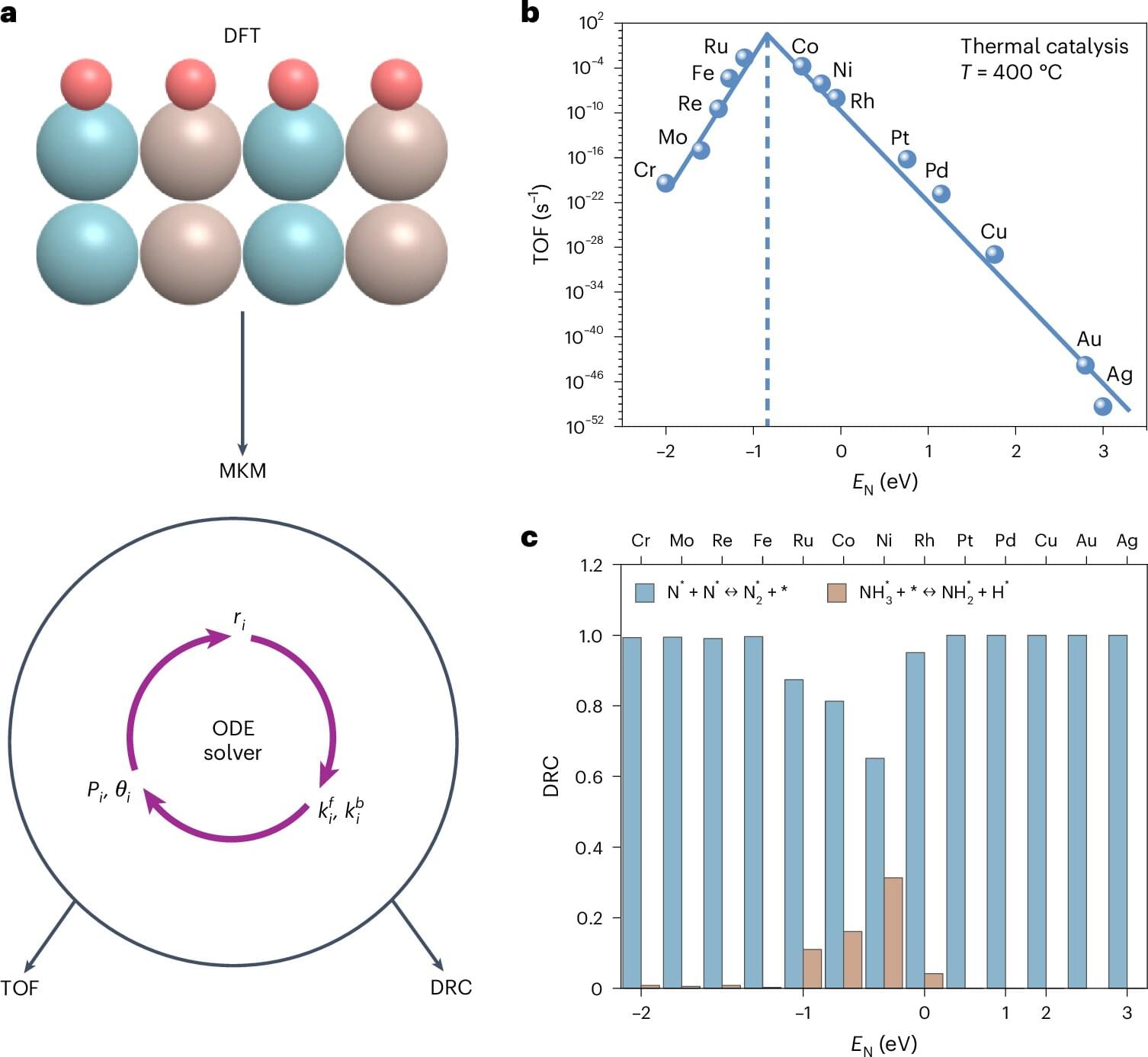
To increase energy efficiency and reduce the carbon footprint of hydrogen fuel production, Fanglin Che, associate professor in the Department of Chemical Engineering at Worcester Polytechnic Institute, is leveraging the power and potential of machine learning and computational modeling. The multi-university team she leads has completed a study that was just published in Nature Chemical Engineering. The study utilized artificial intelligence to identify catalysts with the potential to facilitate cleaner and more efficient hydrogen production.
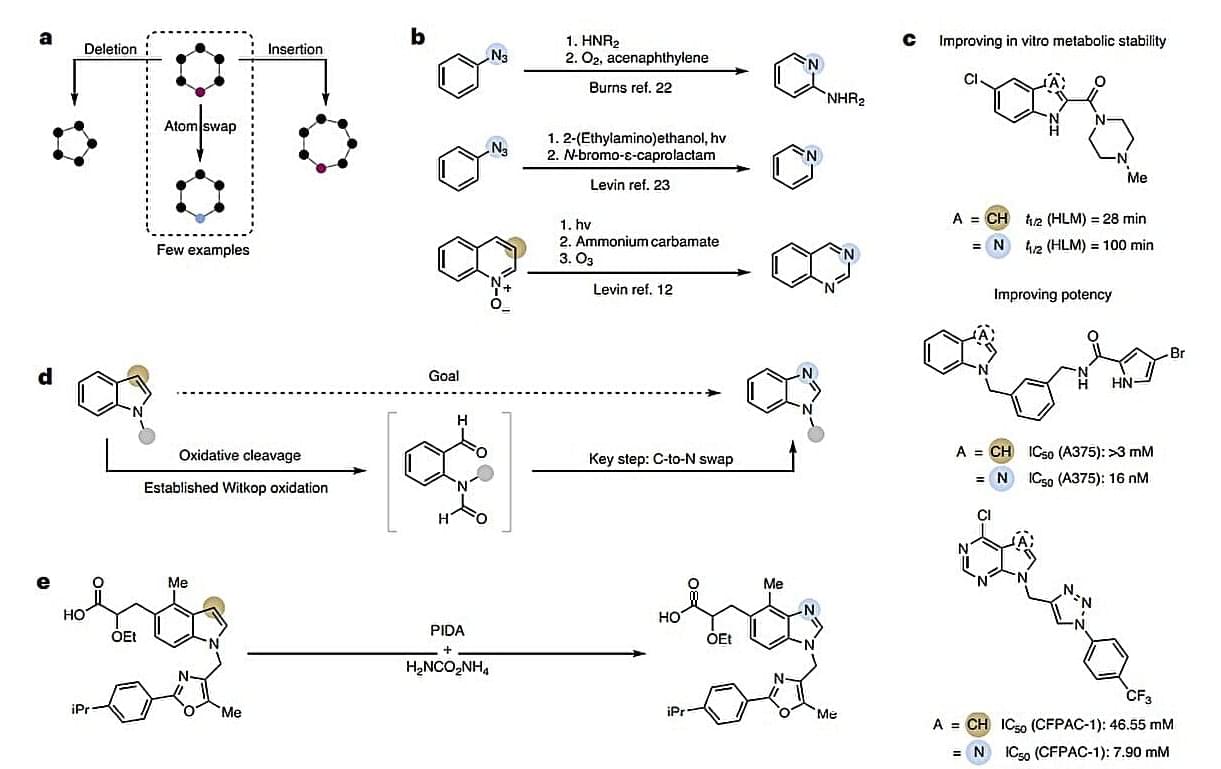
Scientists have achieved a new feat in molecular editing by swapping carbon for nitrogen, enabling the direct conversion of indoles into benzimidazoles. This simple switch in a one-pot method offers a hassle-free and effective way of designing medicinally relevant molecules. The work is published in Nature Chemistry.
Single-atom swap reactions require the selective formation and breaking of multiple bonds at the same time, making them quite rare and challenging.
Researchers from ETH Zurich overcame these hurdles by exploiting the electron-rich indole ring’s eagerness to undergo oxidative cleavage via Witkop oxidation. This step can split the electron-rich ring open to form a dicarbonyl intermediate, thereby creating an entry point for subsequent cascade reactions.
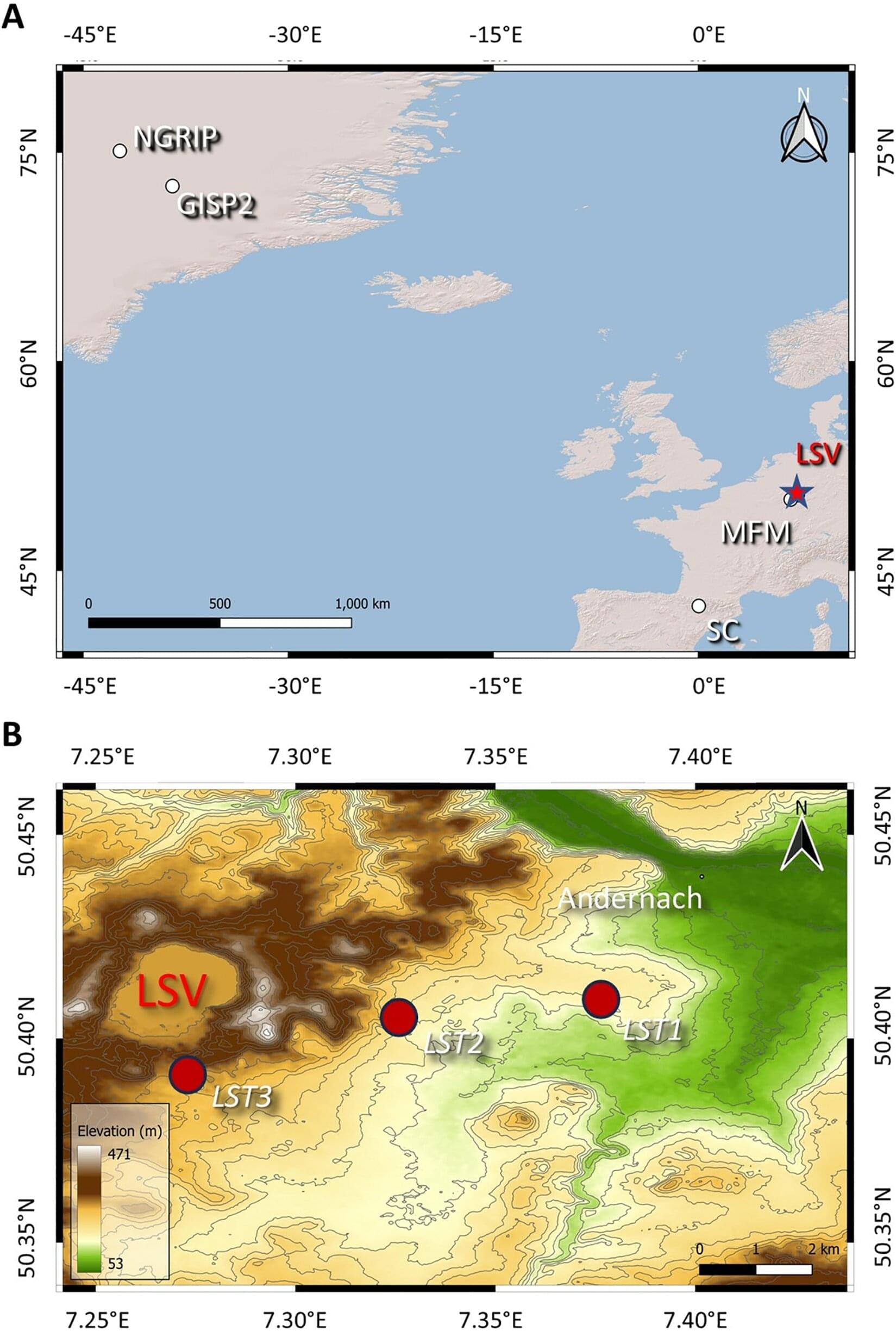
Buried deep in Greenland’s ice sheet lies a puzzling chemical signature that has sparked intense scientific debate. A sharp spike in platinum concentrations, discovered in an ice core (a cylinder of ice drilled out of ice sheets and glaciers) and dated to around 12,800 years ago, has provided support for a hypothesis that Earth was struck by an exotic meteorite or comet at that time.
Our new research published in PLOS One offers a much more mundane explanation: this mystery platinum signature may have originated from a volcanic fissure eruption in Iceland, not space.
The timing matters. The platinum spike occurs near the beginning of our planet’s last great cold period, the Younger Dryas Event. This lasted from about 12,870 to 11,700 years ago and saw temperatures plummet across the northern hemisphere.
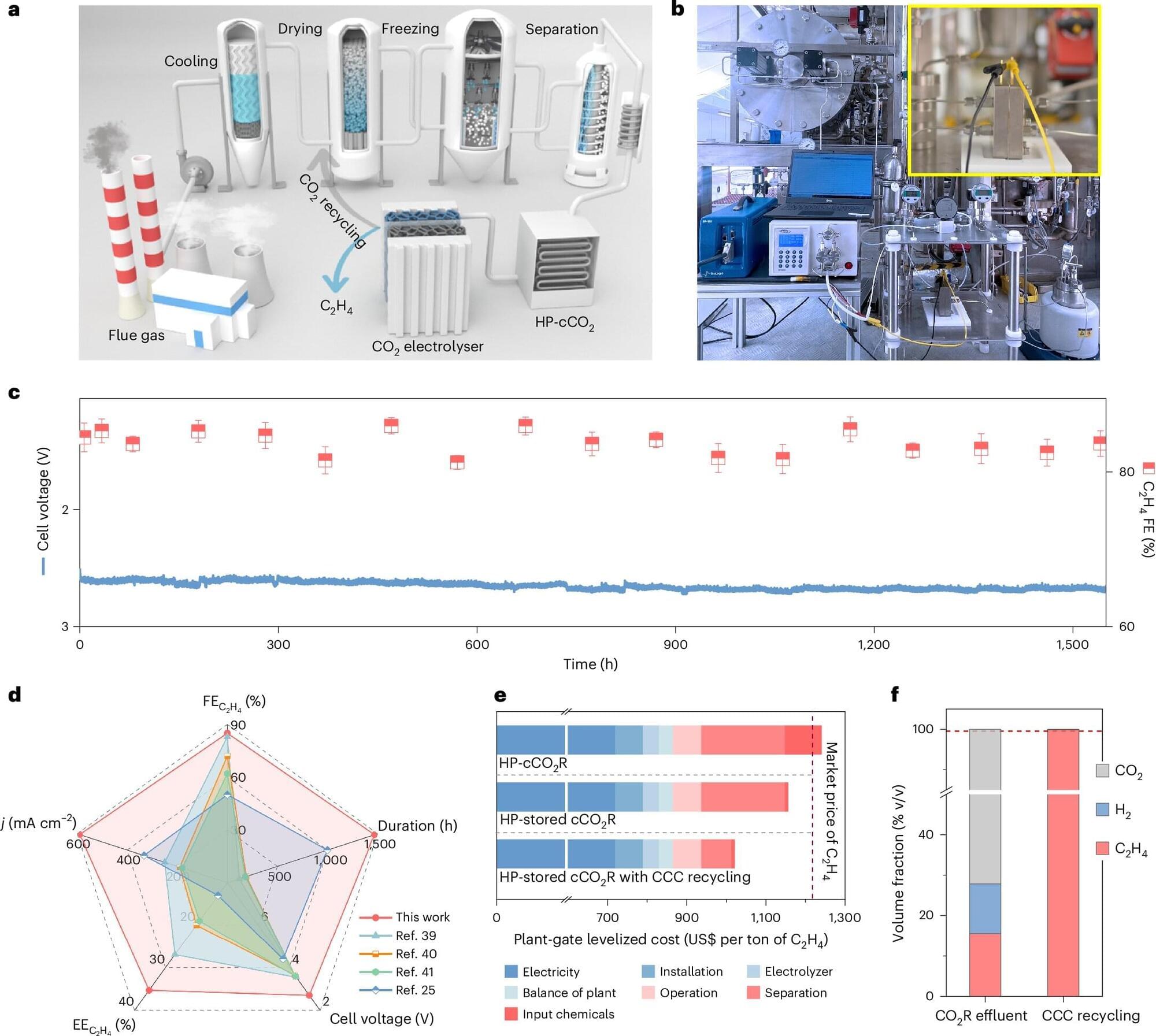
Researchers at King Abdullah University of Science and Technology have unveiled a breakthrough system that could change the way we think about carbon emissions. Published in Nature Catalysis the researchers outline a system for converting captured carbon dioxide (CO₂) into industrial-grade ethylene, a commodity chemical essential to plastics, textiles, and construction. The work shows a direct path to transforming greenhouse gas emissions into valuable chemical products.
In addition to the environmental benefits, lead researcher Assistant Professor Xu Lu said key efficiencies in the system create an opportunity to turn the otherwise costly process of capturing CO2 into a profit.
“We designed and tested the system under realistic industrial conditions using captured, high-pressure CO₂,” he said. “Our results show captured carbon can be valorized into a valuable product with real economic potential.”
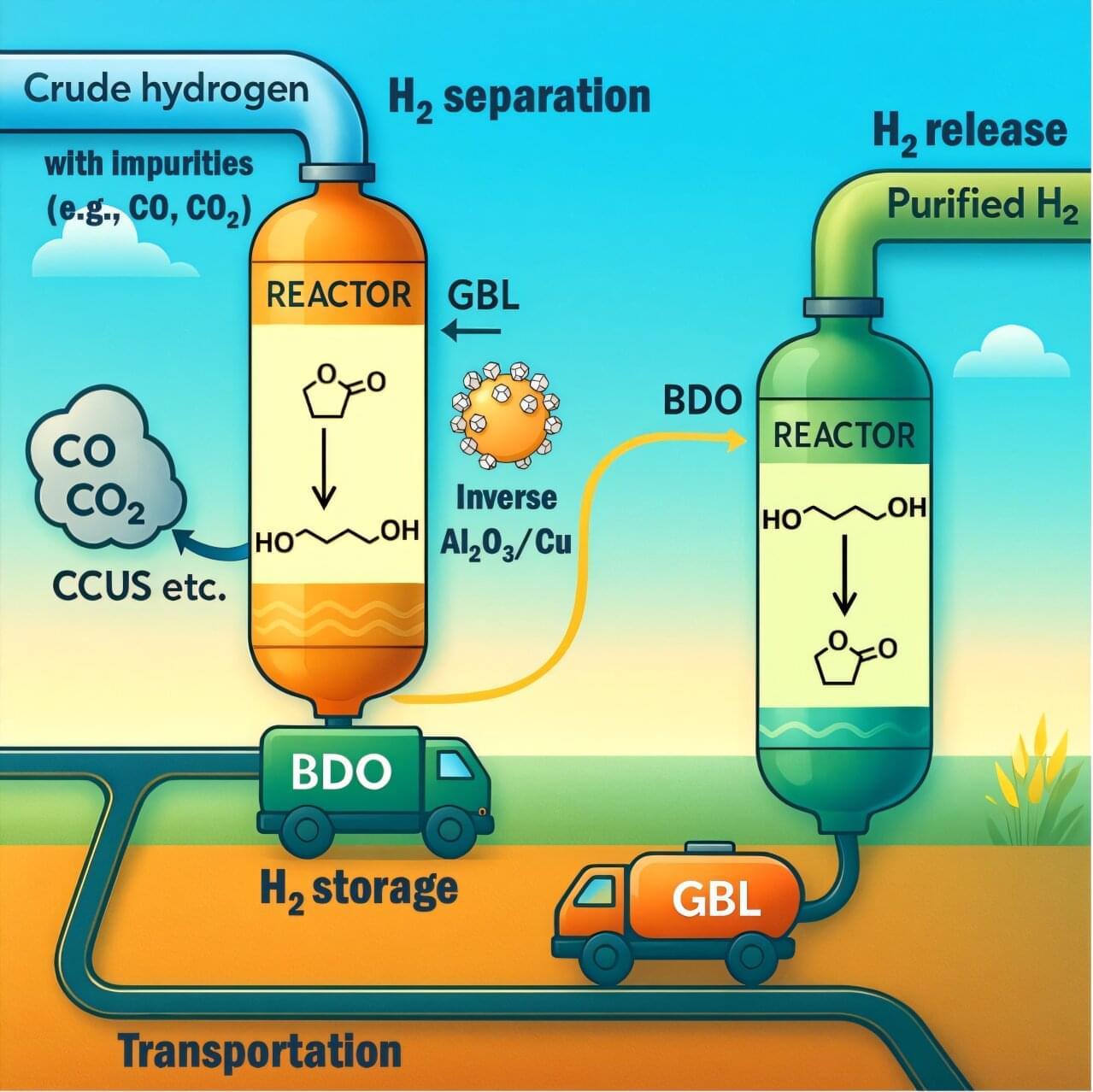
Hydrogen (H2) is an Earth-abundant molecule that is widely used in industrial settings and could soon contribute to the clean generation and storage of electricity. Most notably, it can be used to generate electricity in fuel cells, which could in turn power heavy-duty vehicles or serve as back-up energy systems.
Despite its potential for various real-world applications, hydrogen is often expensive to produce, store and safely transport to desired locations. Moreover, before it can be used, it typically needs to be purified, as hydrogen produced industrially is typically mixed with other gases, such as carbon monoxide (CO), carbon dioxide (CO₂), nitrogen (N₂) and light hydrocarbons.
Researchers at Fudan University and other institutes in China recently devised a new strategy to separate hydrogen from impurities at low temperatures, while also enabling its safe storage and transportation. Their proposed method, outlined in a paper published in Nature Energy, relies on a reversible chemical reaction between two organic compounds that act as hydrogen carriers, enabling the reversible absorption and release of hydrogen.
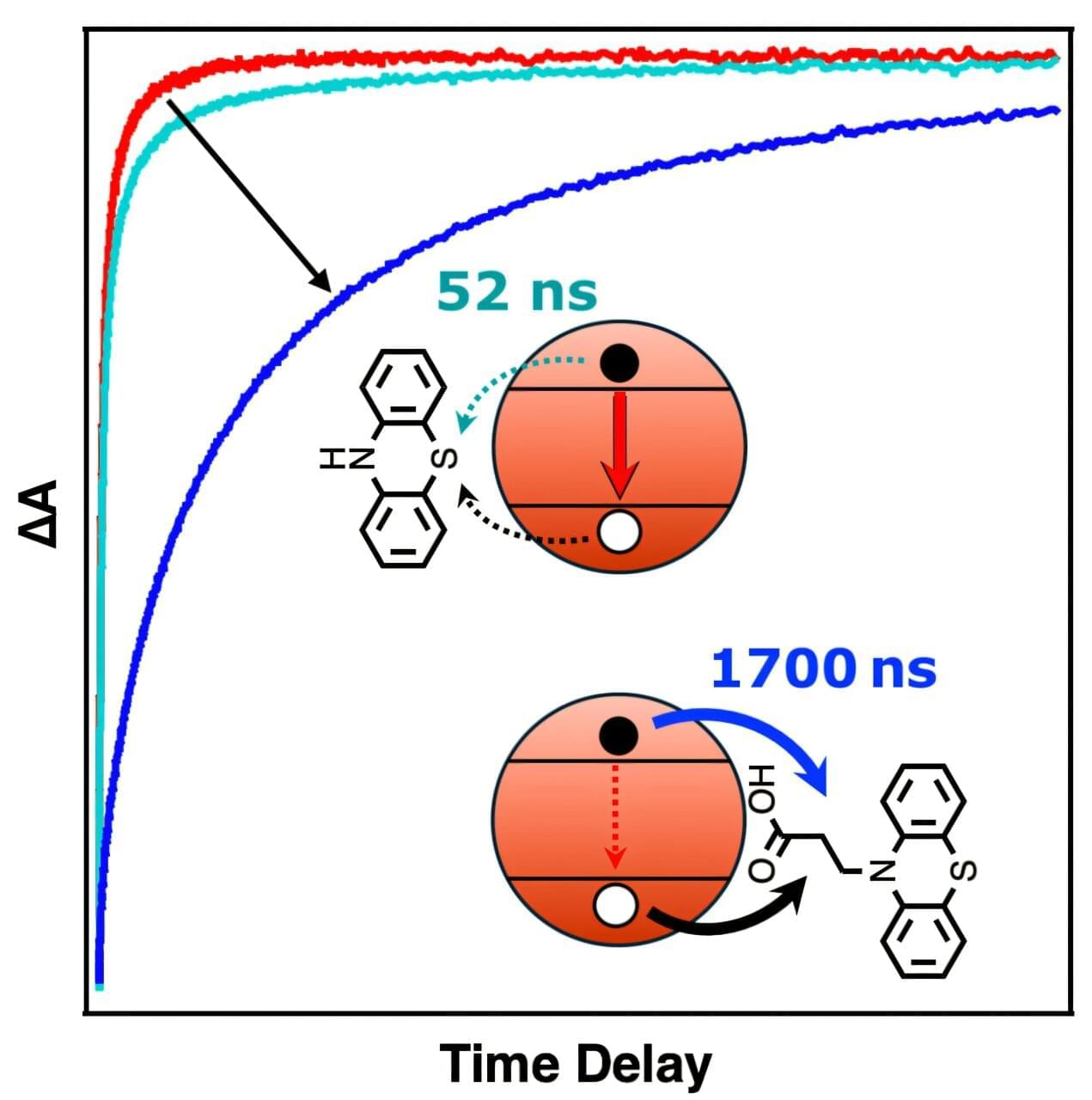
A team of scientists has found a way to slow energy leaks that have impeded the use of tiny nanocrystals in light-driven chemical and energy applications.
As described in an article published in the journal Chem, the team has used a molecule that strongly binds to the nanocrystal’s surface, essentially acting like a dam to hold back the energy stored in the charge-separated state formed after light absorption. This technique extends the lifetime of the charge separation to the longest recorded for these materials, providing a pathway to improved efficiencies and more opportunities to put this energy to work in chemical reactions.
The researchers from the University of Colorado Boulder, the University of California Irvine, and Fort Lewis College were led by RASEI Fellow Gordana Dukovic.
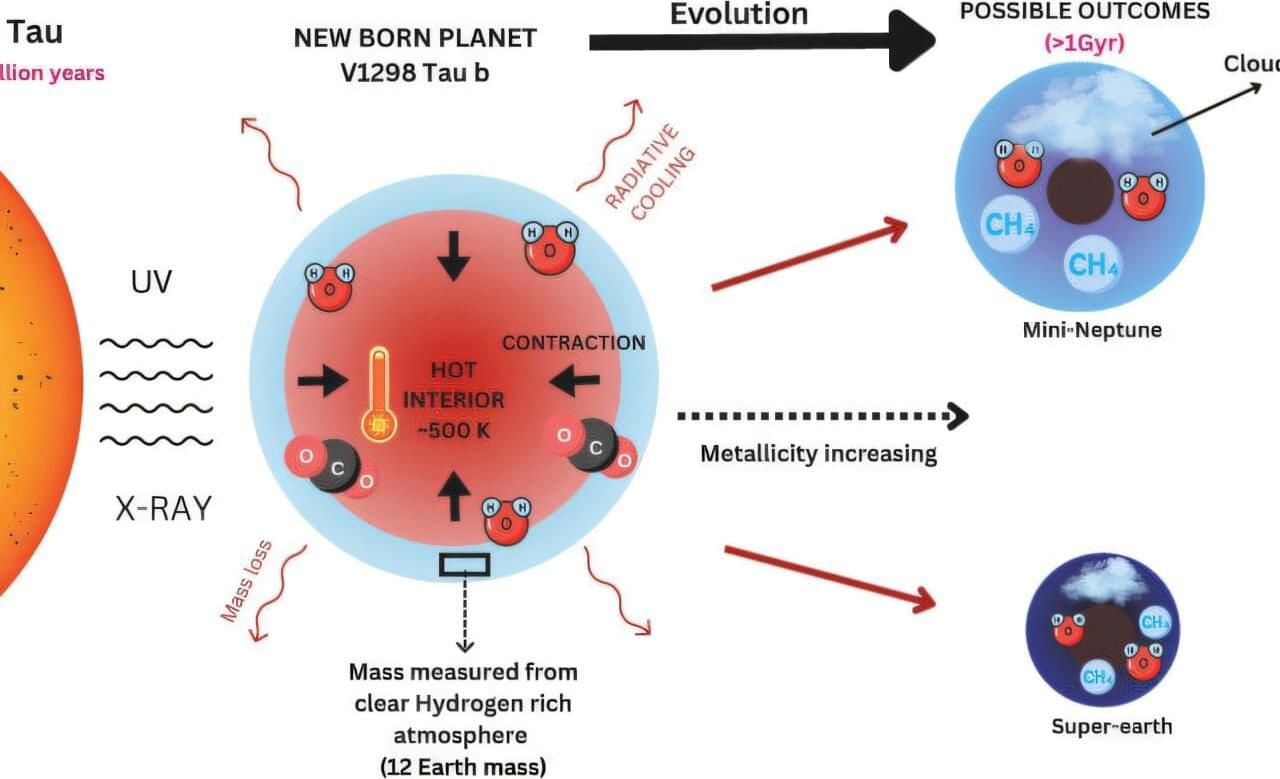
Astronomers have characterized the atmosphere of a young (20 Myr old) transiting exoplanet and found it to be unusually clear and puffy. By analyzing the planet’s atmospheric features, they were able to precisely measure the planet’s mass surpassing traditional dynamical techniques like radial velocity, which poorly perform with such active young stars. They found that V1298 Tau b is a proto-sub-Neptune, still hot and inflated from its recent formation.
The team, led by Saugata Barat (MIT, MA, US) and his Ph.D. supervisor Jean-Michel Désert (UvA, Netherlands) used the James Webb Space Telescope to study the very young planet, and their results are accepted for publication in the Astrophysical Journal and currently available on the preprint server arXiv.
V1298 Tau b is just 10 to 30 million years old and has an unusually clear and puffy atmosphere. The astronomers detected strong absorption signals from molecules like water vapor, methane, carbon dioxide, carbon monoxide, and even hints of complex photochemical processes, such as tentative detections of sulfur dioxide (SO₂) and carbonyl sulfide (OCS).
A research team has discovered an electrochemical method that allows highly selective para-position single-carbon insertion into polysubstituted pyrroles. Their approach has important applications in synthetic organic chemistry, especially in the field of pharmaceuticals.
Their work is published in the Journal of the American Chemical Society on July 14.
“We set out to address the longstanding challenge of achieving single-carbon insertion into aromatic rings with precise positional control,” said Mahito Atobe, Professor, Faculty of Engineering, YOKOHAMA National University. Transformations that modify aromatic rings are central to pharmaceutical and materials synthesis. However, inserting a single carbon atom into a specific position—especially the para-position—has remained extremely rare. Para position describes the location of substituents, those atoms that replace a hydrogen atom on a molecule. In the single carbon insertion approach, researchers add a single carbon atom into a molecule’s carbon framework. This lengthens a carbon chain or expands a ring by one carbon unit.
Method has organic chemistry applications, especially in pharmaceuticals.