Link :
A new study has revealed that Gen X and Millennials are more likely to develop 17 cancer types than other generations.


Brain scans show fasting literally rewires your brain:
Brain scans of participants in a recent study showed changes in brain areas that regulate appetite and addiction, including the inferior frontal orbital gyrus. At the same time, tests of stool samples and blood showed changes in the gut bacteria, especially with types called Coprococcus comes and Eubacterium hallii.
The research was published in Frontiers in Cellular and Infection Microbiology.
The team emphasizes that, not only did the participants lose weight, averaging 7.6 kilograms (16.8 pounds), but there were also noticeable changes in the composition of their gut bacteria, and additional changes in brain regions.
These changes were linked to less activity in a part of the brain called the left inferior frontal orbital gyrus, which helps control food intake. During intermittent fasting, certain beneficial gut bacteria may become more prevalent, producing compounds that influence brain activity related to food intake and impulse control.
This suggests a complex, bidirectional communication between the gut and the brain, where altering the gut environment through fasting can lead to changes in brain function, potentially affecting eating behaviors and decision-making processes related to diet. Intermittent fasting offers multiple benefits, including weight loss, improved metabolic health, enhanced brain function, and potential longevity, by altering eating patterns to incorporate regular periods of fasting.

Diamond, often celebrated for its unmatched hardness and transparency, has emerged as an exceptional material for high-power electronics and next-generation quantum optics. Diamond can be engineered to be as electrically conductive as a metal, by introducing impurities such as the element boron.
Researchers from Case Western Reserve University and the University of Illinois Urbana-Champaign have now discovered another interesting property in diamonds with added boron, known as boron-doped diamonds.
Their findings could pave the way for new types of biomedical and quantum optical devices—faster, more efficient, and capable of processing information in ways that classical technologies cannot. Their results are published in Nature Communications.
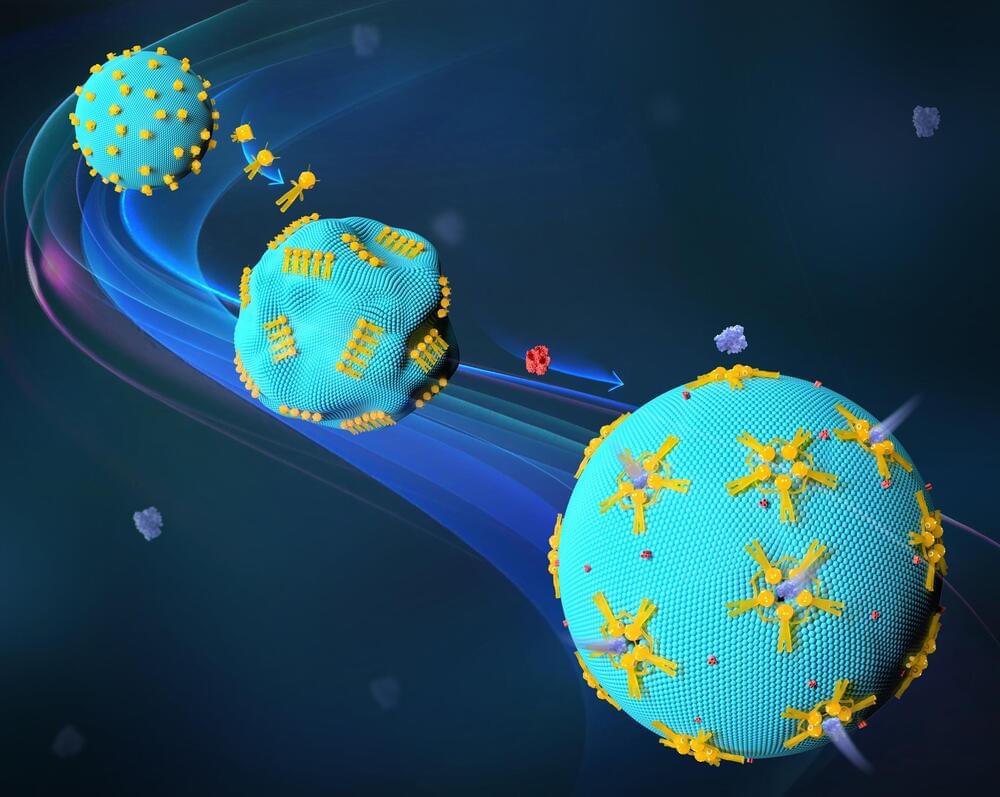
The shape and morphology of a cell play a key role in the biological function. This corresponds to the principle of “form follows function,” which is common in modern fields of design and architecture. The transfer of this principle to artificial cells is a challenge in synthetic biology. Advances in DNA nanotechnology now offer promising solutions. They allow the creation of novel transport channels that are large enough to facilitate the passage of therapeutic proteins across cell membranes.
In this emerging field, Prof. Laura Na Liu, Director of the 2nd Physics Institute at the University of Stuttgart and Fellow at the Max Planck Institute for Solid State Research (MPI-FKF), has developed an innovative tool for controlling the shape and permeability of lipid membranes in synthetic cells. These membranes are made up of lipid bilayers that enclose an aqueous compartment and serve as simplified models of biological membranes. They are useful for studying membrane dynamics, protein interactions, and lipid behavior.
The work is published in Nature Materials.

Researchers at the university of pennsylvania.
The University of Pennsylvania (Penn) is a prestigious private Ivy League research university located in Philadelphia, Pennsylvania. Founded in 1740 by Benjamin Franklin, Penn is one of the oldest universities in the United States. It is renowned for its strong emphasis on interdisciplinary education and its professional schools, including the Wharton School, one of the leading business schools globally. The university offers a wide range of undergraduate, graduate, and professional programs across various fields such as law, medicine, engineering, and arts and sciences. Penn is also known for its significant contributions to research, innovative teaching methods, and active campus life, making it a hub of academic and extracurricular activity.

Researchers from Tokyo Metropolitan University have created nanostructured alumina surfaces which are strongly antibacterial but can be used to culture cells. They found that anodic porous alumina (APA) surfaces prepared using electrochemistry in concentrated sulfuric acid had unprecedented resistance to bacterial growth, but did not hamper cell cultures.
The work is published in the journal Langmuir.
The team’s technology promises to have a big impact on regenerative medicine, where high quality cell cultures without bacterial contamination may be produced without antibiotics.

An unplugged electric instrument may function, but it sounds much better when it is connected to an amplifier. Similarly, toxins and other small molecules at low concentrations in the environment or human body may emit quiet signals that are undetectable without specialized lab technology.
Now, thanks to a “cool trick” in biochemistry used to adapt a sensing platform already being deployed by Northwestern scientists to measure toxins in drinking water, researchers can detect and even measure chemicals at low enough concentrations to have use outside the lab. By attaching circuitry akin to a volume knob to “turn up” weak signals, the team has opened the door for the system to be applied to disease detection and monitoring in the human body for nucleic acids like DNA and RNA, as well as bacteria such as E. coli.
The results, which describe a system that is 10 times more sensitive than previous cell-free sensors built by the team, are published in the journal Nature Chemical Biology.
Introduction: Hyperthermia is an established adjunct in multimodal cancer treatments, with mechanisms including cell death, immune modulation, and vascular changes. Traditional hyperthermia applications are resource-intensive and often associated with patient morbidity, limiting their clinical accessibility. Gold nanorods (GNRs) offer a precise, minimally invasive alternative by leveraging near-infrared (NIR) light to deliver targeted hyperthermia therapy (THT). THT induces controlled tumor heating, promoting immunogenic cell death (ICD) and modulating the tumor microenvironment (TME) to enhance immune engagement. This study explores the synergistic potential of GNR-mediated THT with immunotherapies in immunogenically ‘cold’ tumors to achieve durable anti-tumor immunity.
Methods: GNRs from Sona Nanotech Inc.™ were intratumorally injected and activated using NIR light to induce mild hyperthermia (42–48°C) for 5 minutes. Tumor responses were analyzed for cell death pathways and immune modulation. The immunogenic effects of THT were assessed alone and in combination with intratumoral interleukin-2 (i.t. IL-2) or systemic PD-1 immune checkpoint blockade. Immune cell infiltration, gene expression changes, and tumor growth kinetics were evaluated.
Results: THT reduced tumor burden through cell death mechanisms, including upregulated ICD marked by calreticulin exposure within 48 hours. By 48 hours, CD45+ immune cell levels were increased, including increased levels of immunosuppressive M2 macrophages. While THT led to innate immune cell stimulations highlighted by gene expression upregulation in the STING cGAS pathway and enhanced M1 and dendritic cell levels, tumor regrowth was observed within six days post-treatment. To enhance THT’s immunogenic effects, the therapy was combined with intratumoral interleukin-2 (i.t. IL-2) or systemic PD-1 immune checkpoint blockade. Sequential administration of i.t. IL-2 post-THT induced robust CD8+ T-cell infiltration and led to sustained tumor regression in both treated and distant tumors, accompanied by the emergence of memory T cells. However, IL-2-induced immunosuppressive T-reg populations were also sustained to tumor endpoint suggesting that therapy could be further enhanced.
–
They found that when people with aphantasia try to conjure an image in their mind’s eye, the primary visual cortex – the part of the brain that processes picture-like visual information – is activated, but any images that are produced remain unconscious to the individual.
Published today in Current Biology, opens in a new window, the study, carried out by scientists at UNSW and South China Normal University, used a range of techniques to measure brain activity. Their findings challenge the existing theory that activity in the primary visual cortex directly produces conscious visual imagery.
“People with aphantasia actually do seem to have images of a sort, they remain too weak or distorted to become conscious or be measured by our standard measurement techniques,” says Prof. Joel Pearson, a co-author of the study based at UNSW’s School of Psychology, opens in a new window. “This may be because the visual cortex is wired differently, as evidenced by the data in this new study. This research not only deepens our understanding of the brain but also pushes the boundaries of how we think about imagination and consciousness.”
People with aphantasia still have a blueprint for mental imagery, even if they can’t consciously ‘see’ it.