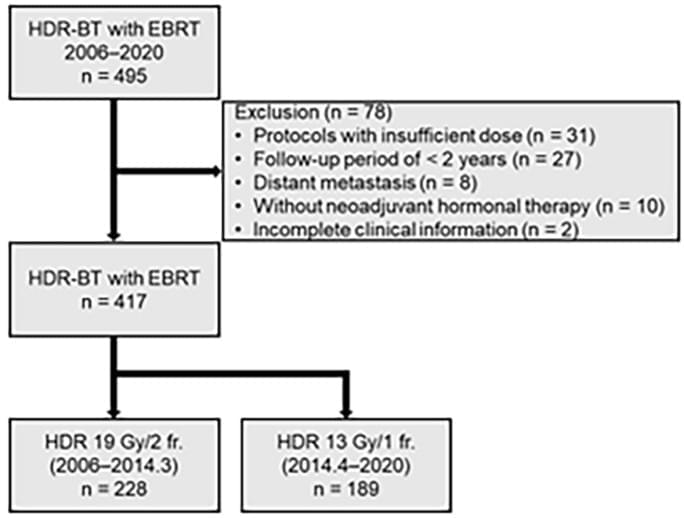
The Automated Intimate Partner Violence Risk Support System (AIRS) utilizes clinical history and radiologic data to pinpoint patients seen in the emergency room who may be at a risk for intimate partner violence (IPV). Developed over the past five years, AIRS has been rolled out to the Brigham and Women’s Hospital’s Emergency Rooms in Boston as well as surrounding primary care sites. Currently, the tool has been validated at the University of California-San Francisco Medical Center and is being evaluated by the Alameda Health System for its role in clinical workflow.
“Data labeling quality is a huge concern—not just with intimate partner violence care, but in machine learning for healthcare and machine learning, broadly speaking,” says cofounder Irene Chen. “Our hope is that with training, clinicians can be taught how to spot intimate partner violence—we are hoping to find a set of cleaner labels.”