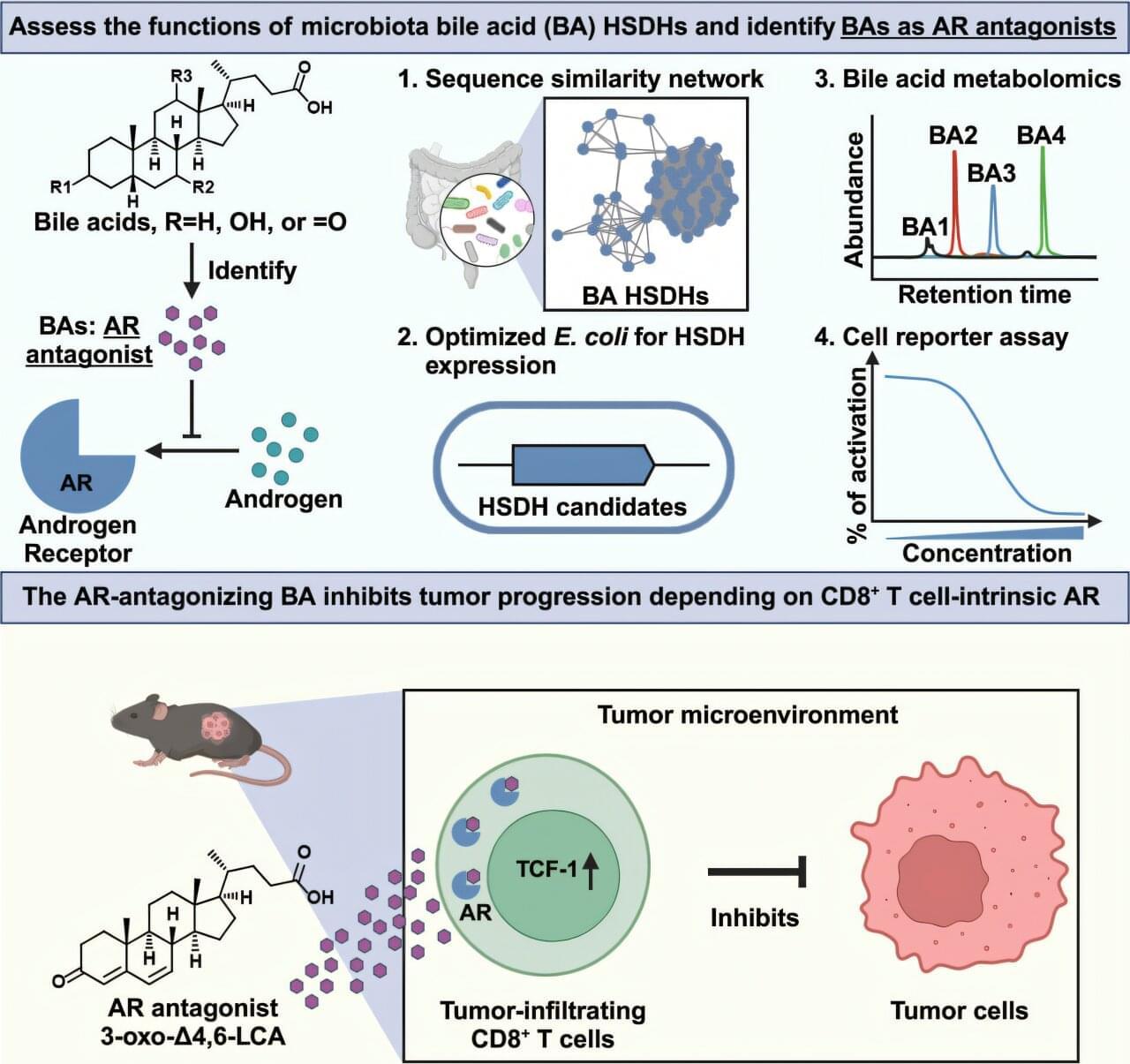
Bacteria naturally present in the human intestine (known as the gut microbiota) can transform cholesterol-derived bile acids into powerful metabolites that strengthen anti-cancer immunity by blocking androgen signaling, according to a preclinical study led by Weill Cornell Medicine investigators. The study was published on April 15 in Cell.
“I was very surprised by our findings. As far as I know, no one has previously discovered molecules like these bile acids that can interact with the androgen receptor in this way,” said co-senior author Dr. Chun-Jun Guo, an associate professor of immunology in medicine in the Division of Gastroenterology and Hepatology and a scientist at the Jill Roberts Institute for Research in Inflammatory Bowel Disease at Weill Cornell Medicine.
Dr. David Artis, director of the Jill Roberts Institute and the Friedman Center for Nutrition and Inflammation and the Michael Kors Professor in Immunology, and Dr. Nicholas Collins, assistant professor of immunology in medicine, both at Weill Cornell Medicine, are co-senior authors of the study. Drs. Wen-Bing Jin, formerly a postdoctoral associate, and Leyi Xiao, a current postdoctoral associate in Dr. Guo’s lab, are the co-first authors of the study.