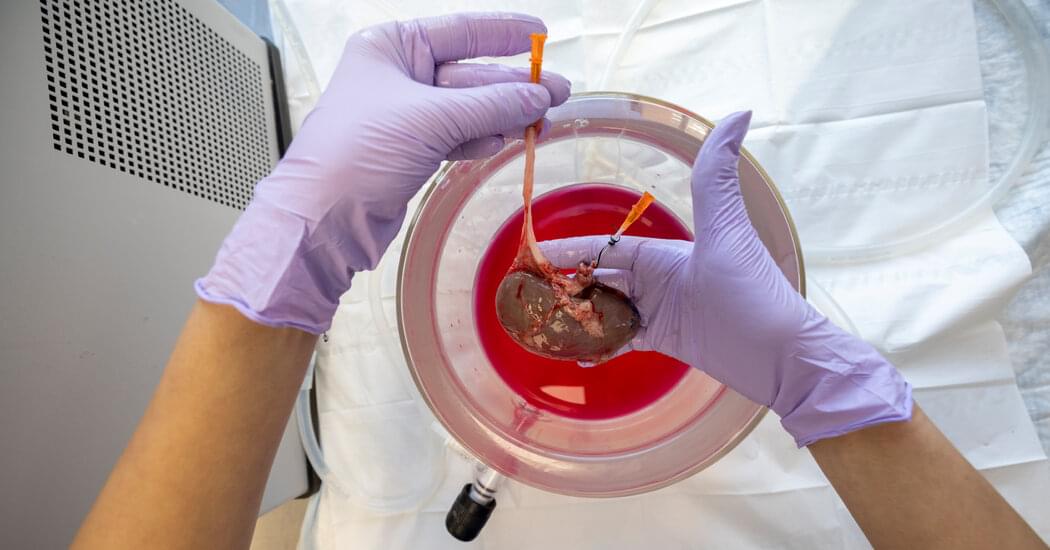
Scientists developed a way to freeze a large mammal’s kidney, which could ease organ shortages in the future. First, they had to see if their method would work in a pig.

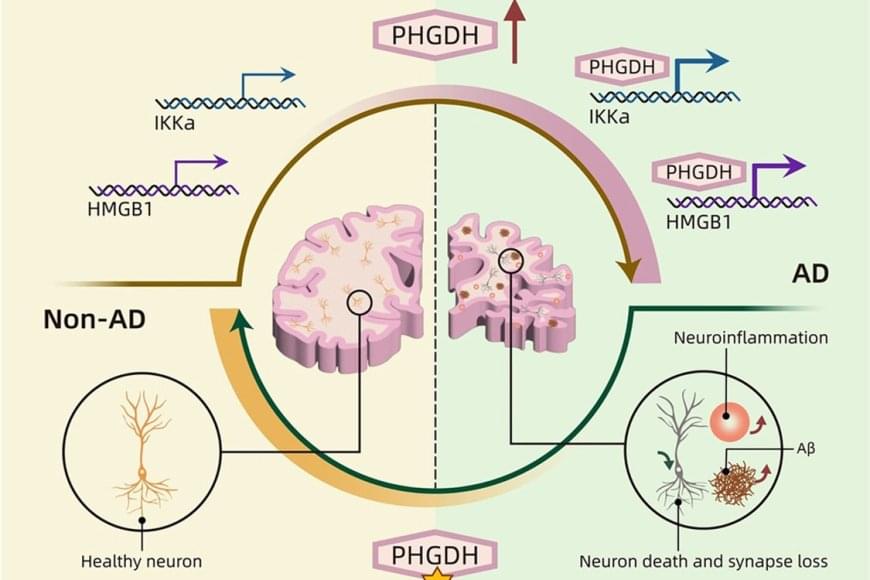
Insomnia, depression, and anxiety are the most common mental disorders. Treatments are often only moderately effective, with many people experiencing returning symptoms. This is why it is crucial to find new leads for treatments. Notably, these disorders overlap a lot, often occurring together. Could there be a shared brain mechanism behind this phenomenon?
Siemon de Lange, Elleke Tissink, and Eus van Someren, together with their colleagues from the Vrije Universiteit Amsterdam, investigated brain scans of more than 40,000 participants from the UK Biobank. The research is published in the journal Nature Mental Health.
Tissink says, “In our lab, we explore the similarities and differences between insomnia, anxiety, and depression. Everyone looks at this from a different perspective: some mainly look at genetics and in this study, we look at brain scans. What aspects are shared between the disorders, and what is unique to each one?”
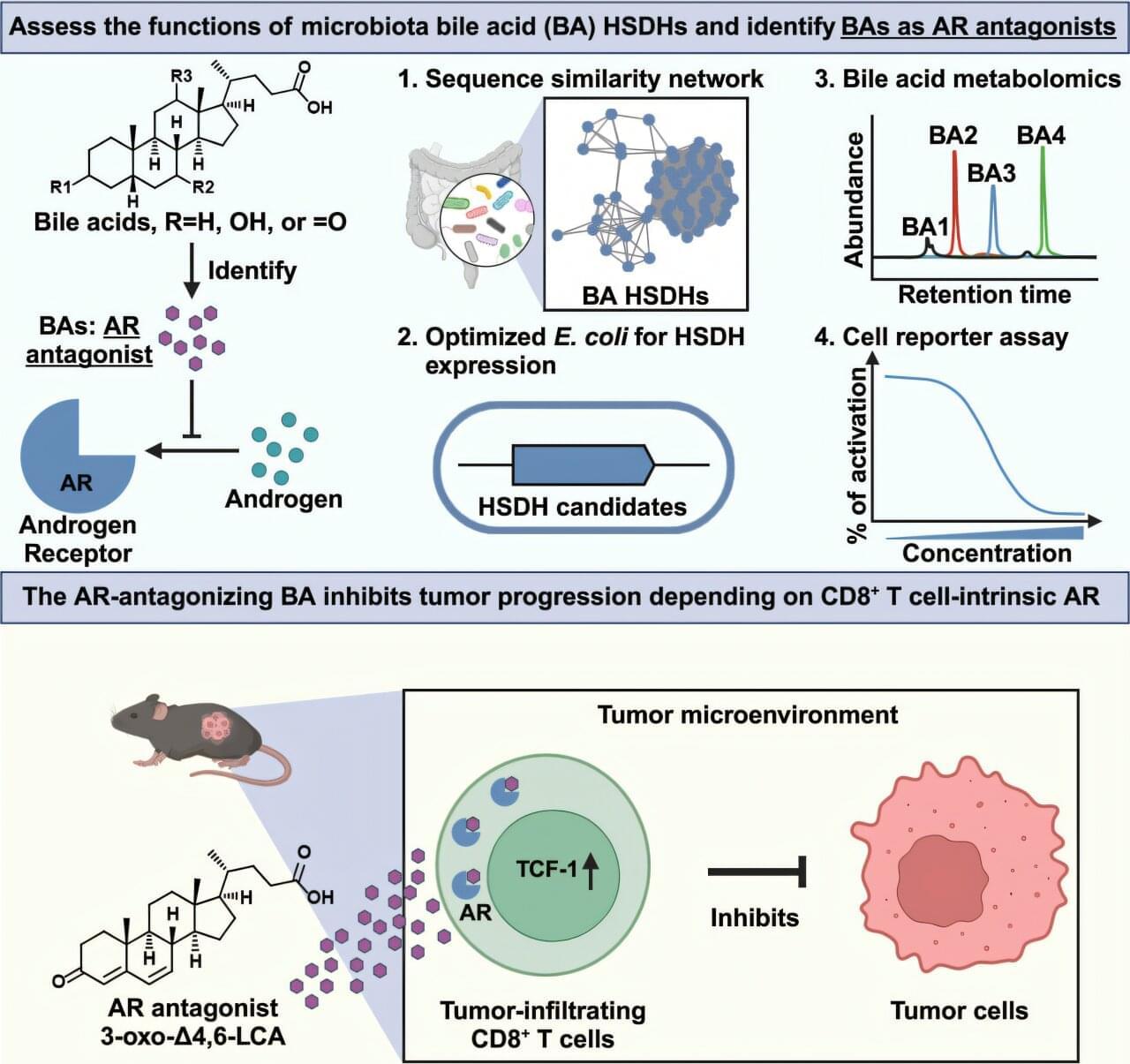
Bacteria naturally present in the human intestine (known as the gut microbiota) can transform cholesterol-derived bile acids into powerful metabolites that strengthen anti-cancer immunity by blocking androgen signaling, according to a preclinical study led by Weill Cornell Medicine investigators. The study was published on April 15 in Cell.
“I was very surprised by our findings. As far as I know, no one has previously discovered molecules like these bile acids that can interact with the androgen receptor in this way,” said co-senior author Dr. Chun-Jun Guo, an associate professor of immunology in medicine in the Division of Gastroenterology and Hepatology and a scientist at the Jill Roberts Institute for Research in Inflammatory Bowel Disease at Weill Cornell Medicine.
Dr. David Artis, director of the Jill Roberts Institute and the Friedman Center for Nutrition and Inflammation and the Michael Kors Professor in Immunology, and Dr. Nicholas Collins, assistant professor of immunology in medicine, both at Weill Cornell Medicine, are co-senior authors of the study. Drs. Wen-Bing Jin, formerly a postdoctoral associate, and Leyi Xiao, a current postdoctoral associate in Dr. Guo’s lab, are the co-first authors of the study.

In our Founder Interview series, we highlight the brightest minds in preventive health, wellness, and longevity. In Episode 6, we’re honored to feature Dr. Emil Kenziorra, founder and CEO at Tomorrow Biostasis —one of the world-leading human cryopreservation experts.
Tell us a little about yourself and your current venture
Doctor and researcher by training, entrepreneur by trade. Longevity has always been my motivation, with a focus on maximal life span extension. I’m running Tomorrow.bio and the non-profit European Biostasis Foundation to push human cryopreservation forward.
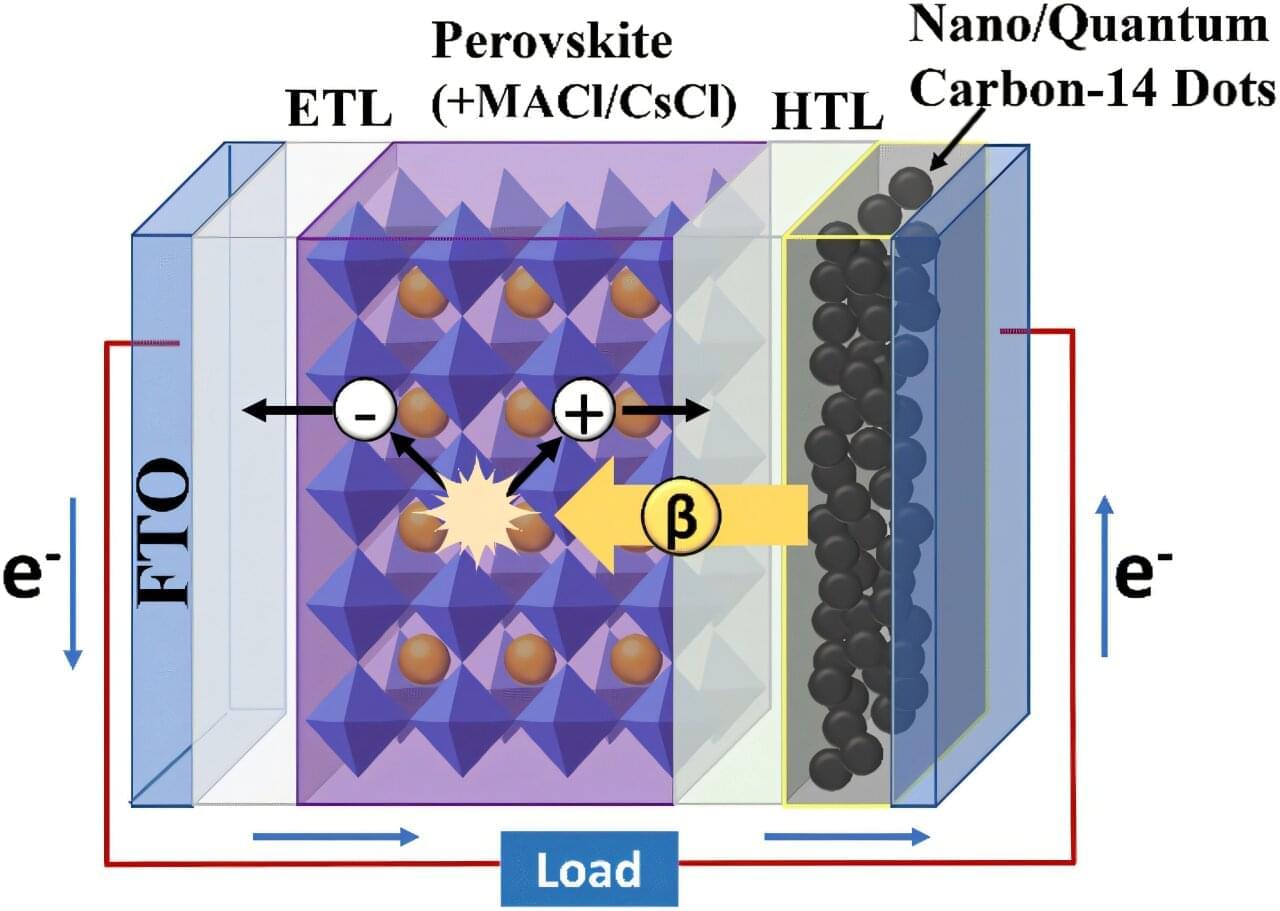
A research team has developed the world’s first next-generation betavoltaic cell by directly connecting a radioactive isotope electrode to a perovskite absorber layer. By embedding carbon-14-based quantum dots into the electrode and enhancing the perovskite absorber layer’s crystallinity, the team achieved both stable power output and high energy conversion efficiency.
The work is published in the journal Chemical Communications. The team was led by Professor Su-Il In of the Department of Energy Science & Engineering at DGIST.
The newly developed technology offers a stable, long-term power supply without the need for recharging, making it a promising next-generation energy solution for fields requiring long-term power autonomy, such as space exploration, implantable medical devices, and military applications.
A watched pot never boils, goes the old saying, but many of us have at least kept an eye on the pot, waiting for the bubbling to start. It’s satisfying to finally see the rolling boil, behind which complex physical mechanisms are at play.
When this happens, the bubbles that form continuously change in shape and size. These dynamic movements influence the surrounding fluid flow, thereby affecting the efficiency of heat transfer from the heat source to the water.
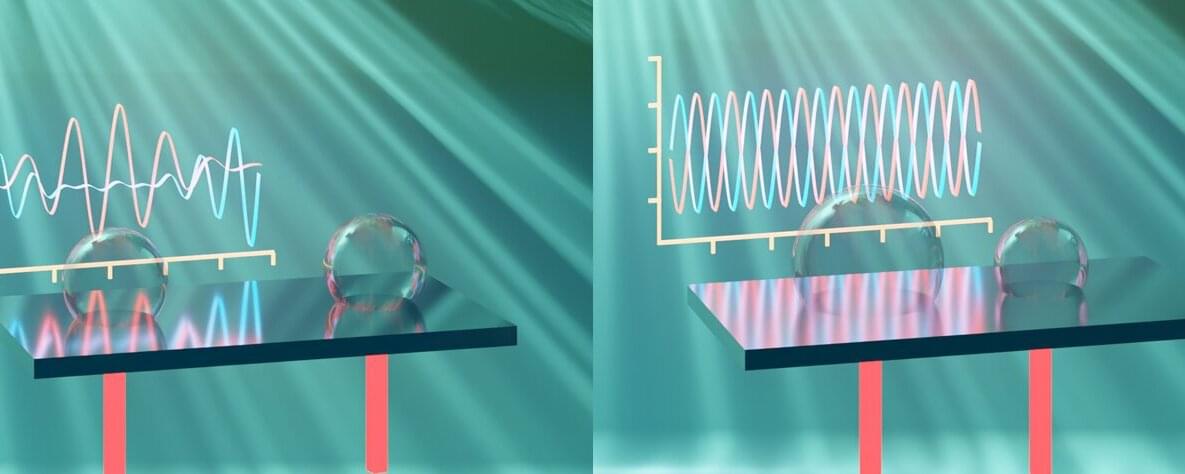
Manipulating small amounts of liquid at high speeds and frequencies is essential for processing large numbers of samples in medical and chemical fields, such as in cell sorting. Microbubble vibrations can create flows and sound waves, aiding in liquid manipulation. However, the collective behavior and interactions of multiple bubbles is poorly understood, so their applications have been limited.
19-year-old Instagram influencer Tilly Lockey has been at the forefront of prosthetic innovation with UK-base…
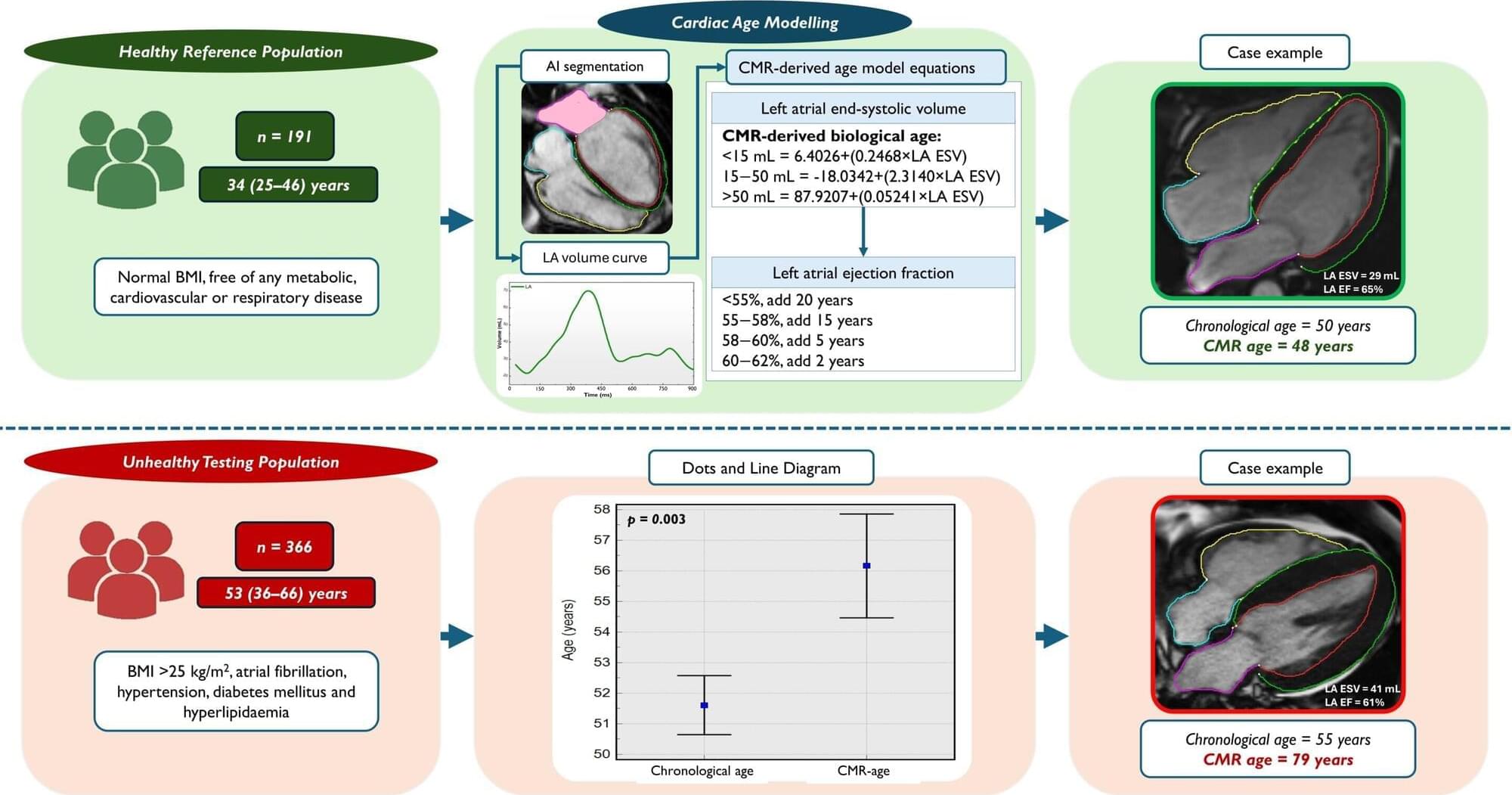
Scientists at the University of East Anglia (UEA) have developed a new way of uncovering the “true age” of a heart using MRI.
Research accepted for publication European Heart Journal Open shows how an MRI scan can reveal your heart’s functional age—and how unhealthy lifestyles can dramatically accelerate this figure. The paper is titled “Cardiac MRI Markers of Ageing: A Multicentre, Cross-sectional Cohort Study.”
It is hoped that the findings could transform how heart disease is diagnosed—offering a lifeline to millions by catching problems before they become deadly.
The duplication and division of cells is critical to keeping all multicellular organisms alive. But the opposite process is equally important: cell death. Controlled death of cells, or programmed cell death, is also necessary for the proper development and function of the body. It has also been a focus of researchers developing treatments for cancer by finding ways to activate the cell death of cancer cells themselves.
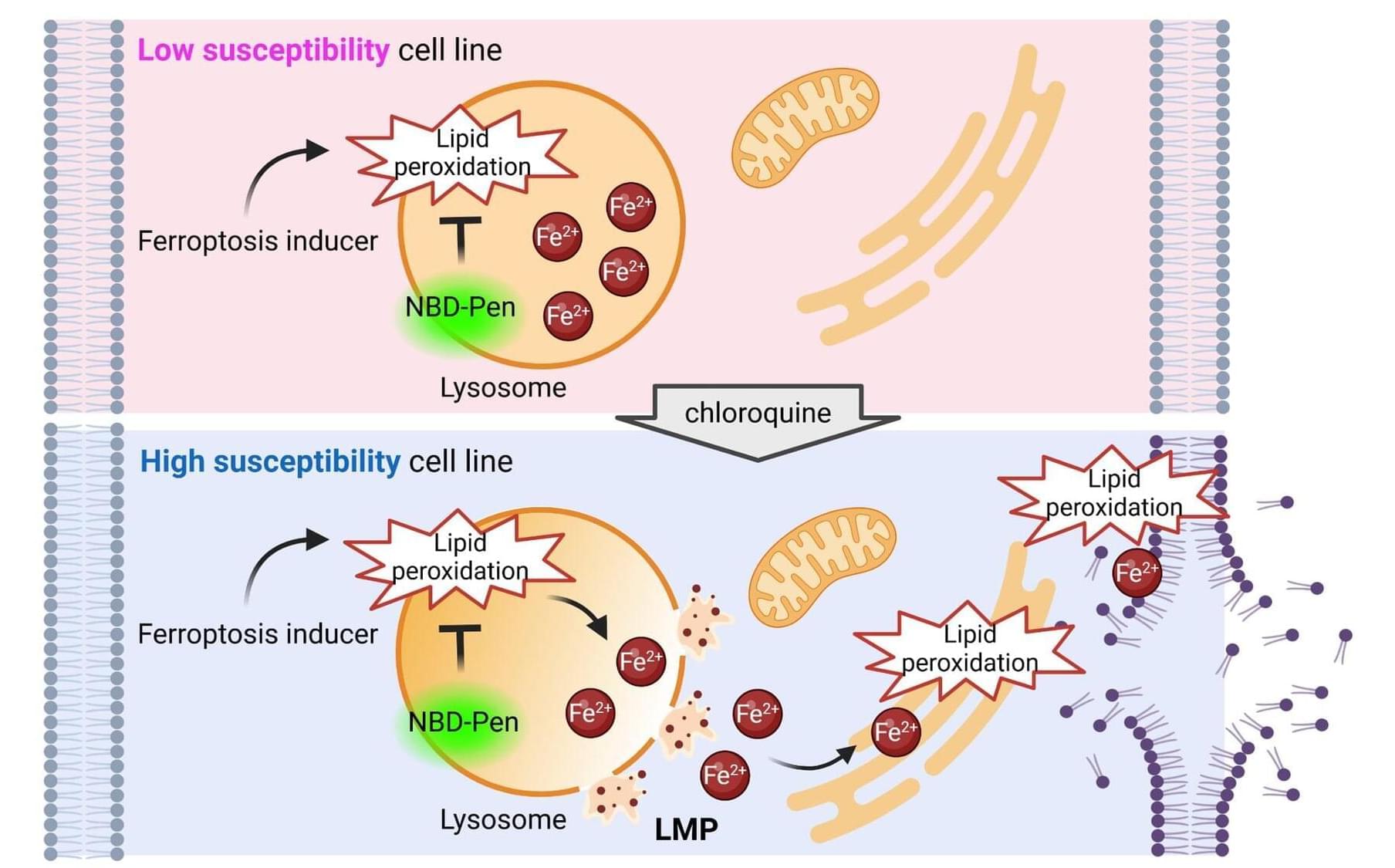
Ferroptosis is a recently discovered form of programmed cell death and has been a promising target for the development of cancer treatments. It is mediated by iron molecules, with the cell dying through the degradation of the phospholipid bilayer by oxidation, a process called lipid peroxidation. However, recent studies have shown that certain cancer cells are less susceptible to ferroptosis, raising concerns that this resistance could pose a barrier to future therapeutics.
In a paper published in Nature Communications, researchers from Kyushu University, using cultured cells and mice, found that the lipid peroxidation of the lysosomes—the organelle responsible for degrading and recycling molecules in a cell—plays a critical role in the execution of ferroptosis.

Antimicrobial resistance (AMR) presents a serious challenge in today’s world. The use of antimicrobials (AMU) significantly contributes to the emergence and spread of resistant bacteria. Companion animals gain recognition as potential reservoirs and vectors for transmitting resistant microorganisms to both humans and other animals. The full extent of this transmission remains unclear, which is particularly concerning given the substantial and growing number of households with companion animals. This situation highlights critical knowledge gaps in our understanding of risk factors and transmission pathways for AMR transfer between companion animals and humans. Moreover, there’s a significant lack of information regarding AMU in everyday veterinary practices for companion animals. The exploration and development of alternative therapeutic approaches to antimicrobial treatments of companion animals also represents a research priority. To address these pressing issues, this Reprint aims to compile and disseminate crucial additional knowledge. It serves as a platform for relevant research studies and reviews, shedding light on the complex interplay between AMU, AMR, and the role of companion animals in this global health challenge. This Reprint is especially addressed to companion animal veterinary practitioners as well as all researchers working on the field of AMR in both animals and humans, from a One Health perspective.