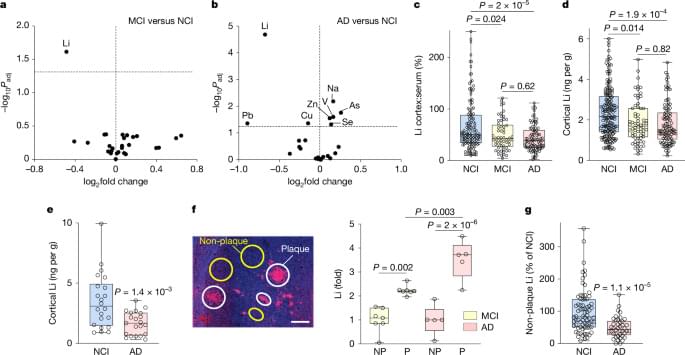
Eating pistachios every night for 12 weeks altered bacteria in the gut, according to new study. A new study reveals that swapping a typical nighttime carbohydrate snack for pistachios may beneficially alter gut bacteria in people with prediabetes. Conducted by Penn State researchers, the 12-week clinical trial found that pistachio consumption increased beneficial gut microbes like Roseburia and reduced harmful ones such as Blautia hydrogenotrophica. These microbiome changes could potentially support metabolic health and slow the progression to Type 2 diabetes. While more research is needed to confirm health outcomes, this study positions pistachios as a promising late-night snack with microbiome-boosting potential.
Prediabetes affects a third of people in the United States and most of them will develop Type 2 diabetes, yet effective dietary intervention strategies remain limited. Pistachios have shown promise in improving markers of diet quality, yet little is known about how they influence the gut microbiome — a key player in glucose regulation and inflammation.
A new study led by Kristina Petersen, associate professor of nutritional sciences at Penn State, determined that nighttime pistachio consumption affects gut bacteria in adults with prediabetes. Though the potential therapeutic implications of the findings remain unclear, according to Petersen, they may prove significant for people who are working to improve their metabolic health.