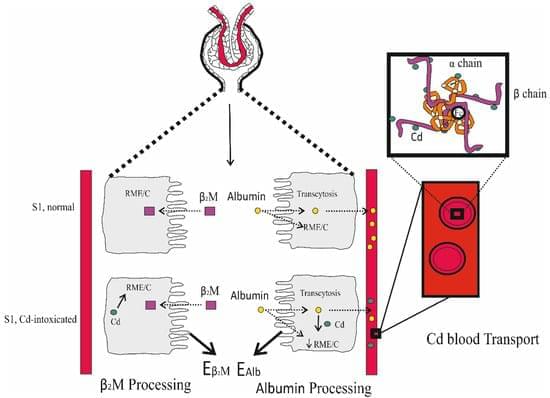
Cadmium (Cd) is a metal with no nutritional value or physiological role. However, it is found in the body of most people because it is a contaminant of nearly all food types and is readily absorbed. The body burden of Cd is determined principally by its intestinal absorption rate as there is no mechanism for its elimination. Most acquired Cd accumulates within the kidney tubular cells, where its levels increase through to the age of 50 years but decline thereafter due to its release into the urine as the injured tubular cells die. This is associated with progressive kidney disease, which is signified by a sustained decline in the estimated glomerular filtration rate (eGFR) and albuminuria. Generally, reductions in eGFR after Cd exposure are irreversible, and are likely to decline further towards kidney failure if exposure persists.