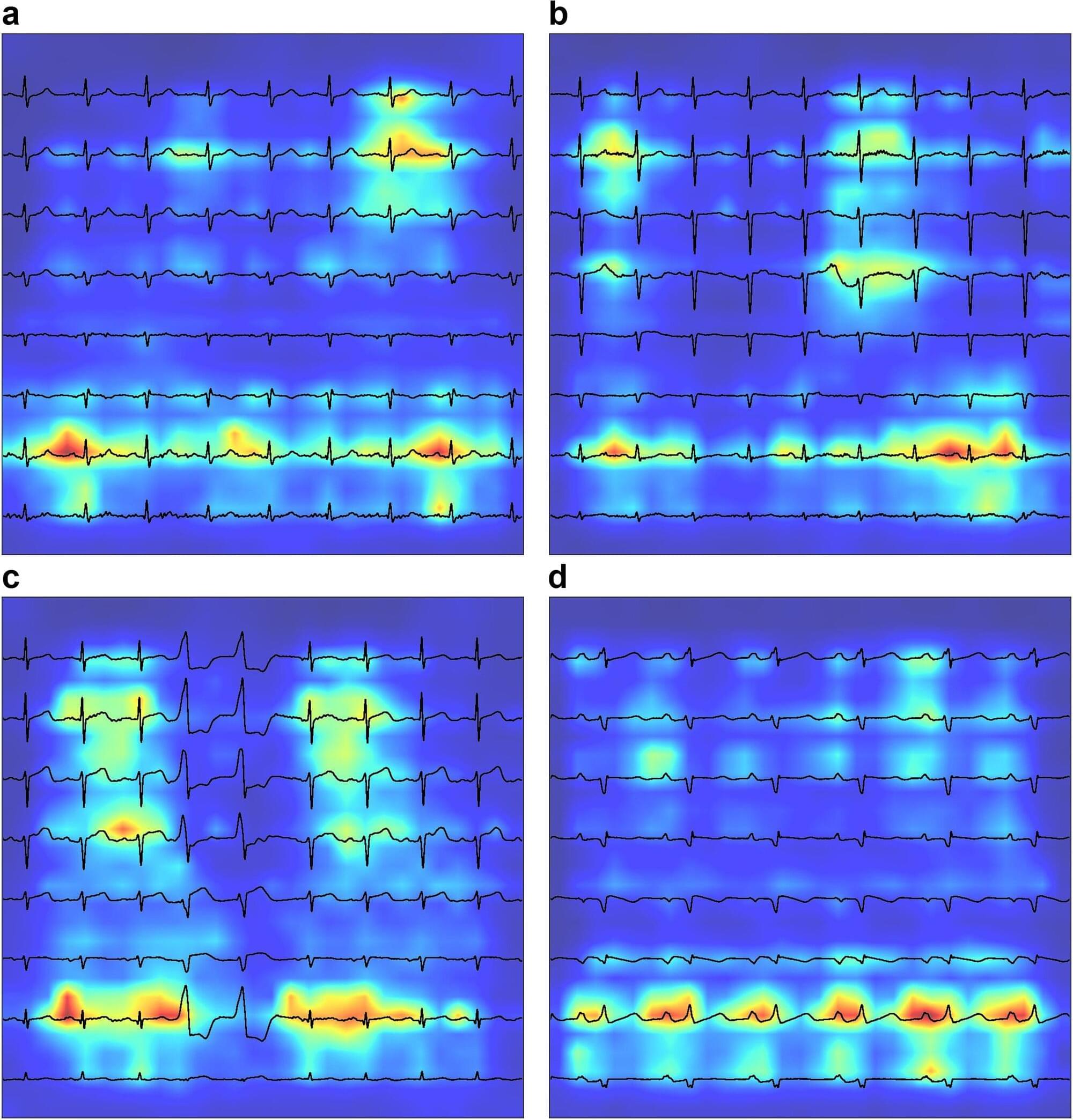
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality globally. Effective management hinges on early diagnosis, which is often impeded by non-specific symptoms and resource-intensive diagnostic methods. A study published in the journal eBioMedicine assesses the effectiveness of electrocardiograms (ECGs) analyzed via deep learning as a tool for early COPD detection.
Mount Sinai researchers utilized a Convolutional Neural Network model to analyze ECGs, or medical tests that record the heart’s electrical activity, and can detect COPD. The primary outcome was the accuracy of a new clinical COPD diagnosis, as determined by International Classification of Disease codes. They performed an evaluation using Area-Under-the-Curve (AUC) metrics, derived by testing against ECGs from patients at five hospitals within the Mount Sinai Health System who represented a demographically diverse patient population in New York City.
They examined data from 2006 to 2023 within the GE MUSE system that exports electrocardiograms as individual XML files containing raw waveforms. The experts also used ECGs from patients at another hospital and ECGs of patients with COPD within the UK BioBank to expand the cohort and validate the analysis.