Category: biotech/medical – Page 1,441


A “Nano-Robot” Built Entirely from DNA to Explore Cell Processes
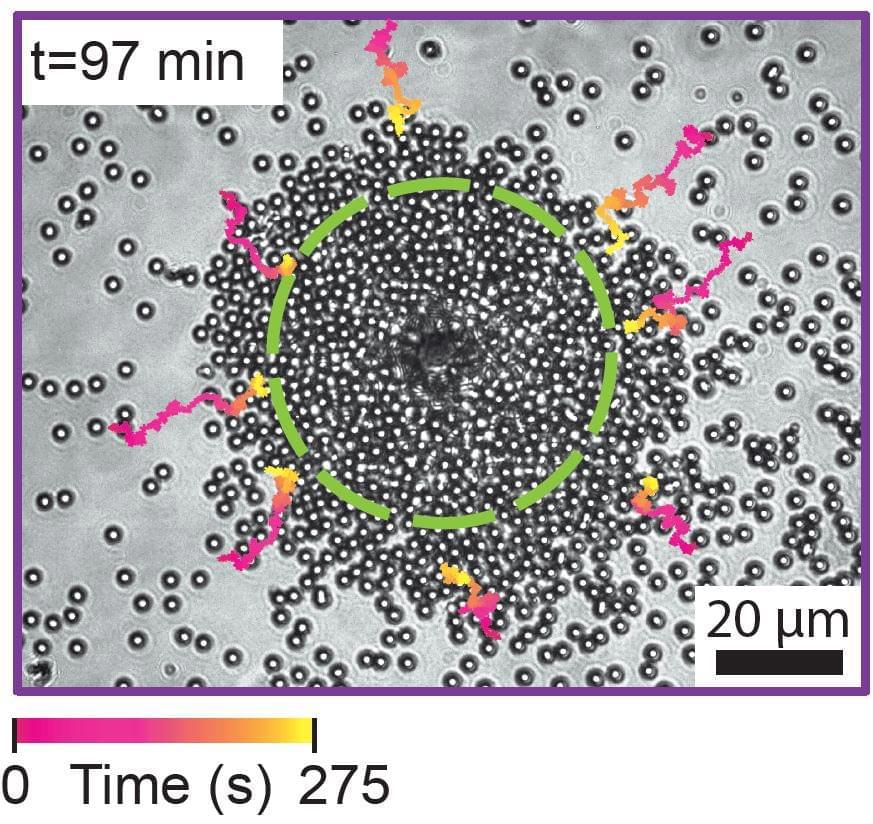
Constructing a tiny robot from DNA and using it to study cell processes invisible to the naked eye… You would be forgiven for thinking it is science fiction, but it is in fact the subject of serious research by scientists from Inserm, CNRS and Université de Montpellier at the Structural Biology Center in Montpellier[1]. This highly innovative “nano-robot” should enable closer study of the mechanical forces applied at microscopic levels, which are crucial for many biological and pathological processes. It is described in a new study published in Nature Communications.
Our cells are subject to mechanical forces exerted on a microscopic scale, triggering biological signals essential to many cell processes involved in the normal functioning of our body or in the development of diseases.
For example, the feeling of touch is partly conditional on the application of mechanical forces on specific cell receptors (the discovery of which was this year rewarded by the Nobel Prize in Physiology or Medicine).
Another byproduct of aging: Hypermutations in the brain
Throughout life, cells of the body acquire somatic mutations. In frozen post-mortem human brains, researchers from Yale University have found that somatic, or non-inherited, mutations are considerably more likely to accumulate in roughly 6% of brains, and these “hypermutable” brains are typically 40 years of age or older.
The behavior, comparable to clonal hematopoiesis in the bone marrow that can result in blood cancer in elderly people, is attributed to cell lines with mutations that outcompete other cell lines.
This is the first large-scale study of somatic mutations in human brains. Scientists were not expecting to find this hypermutability in older populations.

Scientists discover new ‘origins of life’ chemical reactions
Four billion years ago, the Earth looked very different than it does today, devoid of life and covered by a vast ocean. Over the course of millions of years, in that primordial soup, life emerged. Researchers have long theorized how molecules came together to spark this transition. Now, scientists at Scripps Research have discovered a new set of chemical reactions that use cyanide, ammonia and carbon dioxide—all thought to be common on the early earth—to generate amino acids and nucleic acids, the building blocks of proteins and DNA.
“We’ve come up with a new paradigm to explain this shift from prebiotic to biotic chemistry,” says Ramanarayanan Krishnamurthy, Ph.D., an associate professor of chemistry at Scripps Research, and lead author of the new paper, published July 28, 2022 in the journal Nature Chemistry. “We think the kind of reactions we’ve described are probably what could have happened on early earth.”
In addition to giving researchers insight into the chemistry of the early earth, the newly discovered chemical reactions are also useful in certain manufacturing processes, such as the generation of custom labeled biomolecules from inexpensive starting materials.
“Life-Like” Lasers Can Self-Organize, Adapt, and Cooperate Like Living Systems
Self-organizing lasers could lead to new materials for sensing, computing, light sources, and displays by mimicking features of living systems.
Although many artificial materials have advanced properties, they have a long way to go to combine the versatility and functionality of living materials that can adapt to their situation. For example, in the human body bone and muscle continuously reorganize their structure and composition to better sustain changing weight and level of activity.
Now, scientists have demonstrated the first spontaneously self-organizing laser device, which can reconfigure when conditions change.

Human-like behavioral variability blurs the distinction between a human and a machine in a nonverbal Turing test
Human-like temporal variability in movements is a powerful hint that humans use to ascribe humanness to robots.
A team of researchers at the University of Geneva has found that ketamine is unlikely to be addictive to people who use it for extended periods of time. In their paper published in the journal Nature, the group describes their study of the impact of the synthetic compound on the brains of mice and what they learned about its impact on different brain regions. Rianne Campbell and Mary Kay Lobo, with the University of Maryland School of Medicine have published a News and Views piece in the same journal issue outlining the work done by the team in Switzerland.
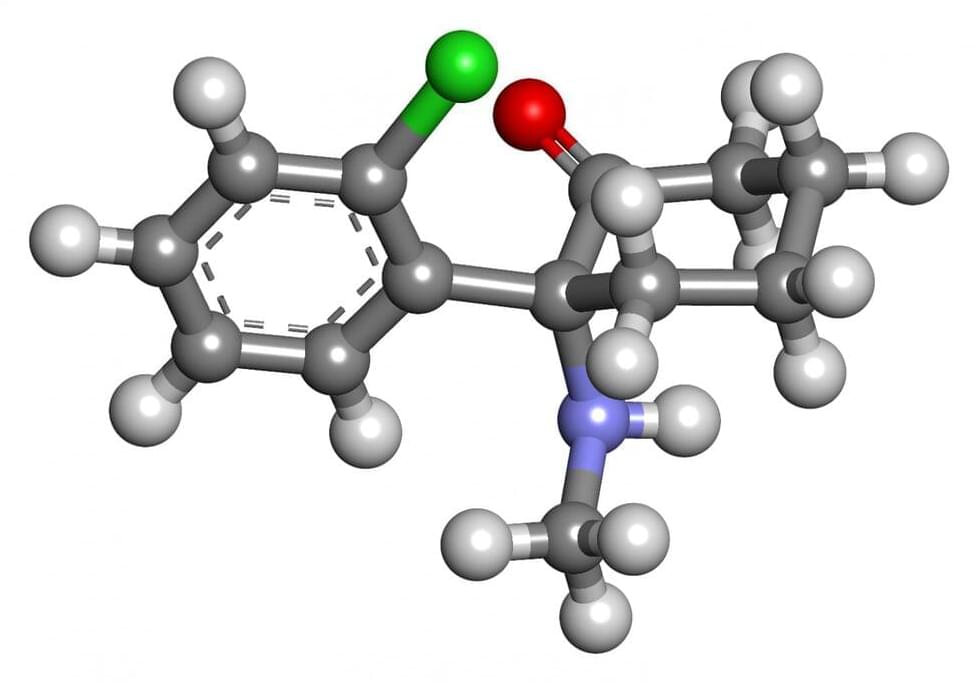
Ketamine found to be unlikely to lead to addiction
A team of researchers at the University of Geneva has found that ketamine is unlikely to be addictive to people who use it for extended periods of time. In their paper published in the journal Nature, the group describes their study of the impact of the synthetic compound on the brains of mice and what they learned about its impact on different brain regions. Rianne Campbell and Mary Kay Lobo, with the University of Maryland School of Medicine have published a News and Views piece in the same journal issue outlining the work done by the team in Switzerland.

Flowers can hear buzzing bees—and it makes their nectar sweeter
This single study has cracked open an entirely new field of scientific research, which Hadany calls phytoacoustics.
Veits wants to know more about the underlying mechanisms behind the phenomenon the research team observed. For instance, what molecular or mechanical processes are driving the vibration and nectar response? She also hopes the work will affirm the idea that it doesn’t always take a traditional sense organ to perceive the world.
“Some people may think, How can [plants] hear or smell?” Veits says. “I’d like people to understand that hearing is not only for ears.”