New, ultrafast and energy-efficient, light-based communication networks could make remote robots a reality — with huge implications for surgery, construction and logistics.



Scientists have uncovered a breakthrough in the fight against a rare genetic condition that causes children to age much faster than normal. The discovery involves “longevity genes” found in people who live exceptionally long lives, often beyond 100 years. Researchers from the University of Bristol and IRCCS MultiMedica found that these genes, which help maintain the health of the heart and blood vessels during aging, could reverse some of the damage caused by this devastating disease.
The study, published in Signal Transduction and Targeted Therapy, is the first to show that a gene from long-lived individuals can slow down heart aging in a model of Progeria. Known scientifically as Hutchinson-Gilford Progeria Syndrome (HGPS), this rare and fatal disorder causes children to exhibit signs of “accelerated aging.”
Progeria stems from a mutation in the LMNA gene, which leads to the creation of a harmful protein called progerin. This protein disrupts normal cell function, particularly in the heart and blood vessels. Most affected children die in their teenage years from heart complications, though some, like Sammy Basso — the oldest known person with Progeria — live longer. Sammy passed away on October 24, 2024, at the age of 28.
Researchers at ETH Zurich have developed artificial muscles that contain microbubbles and can be controlled with ultrasound. In the future, these muscles could be deployed in technical and medical settings as gripper arms, tissue patches, targeted drug delivery, or robots.
It might look like a simple material experiment at first glance, as a brief ultrasound stimulation induces a thin strip of silicone to start bending and arching. But that’s just the beginning. A team led by Daniel Ahmed, Professor of Acoustic Robotics for Life Sciences and Healthcare, has developed a new class of artificial muscles: flexible membranes that respond to ultrasound with the help of thousands of microbubbles.
The work is published in the journal Nature.
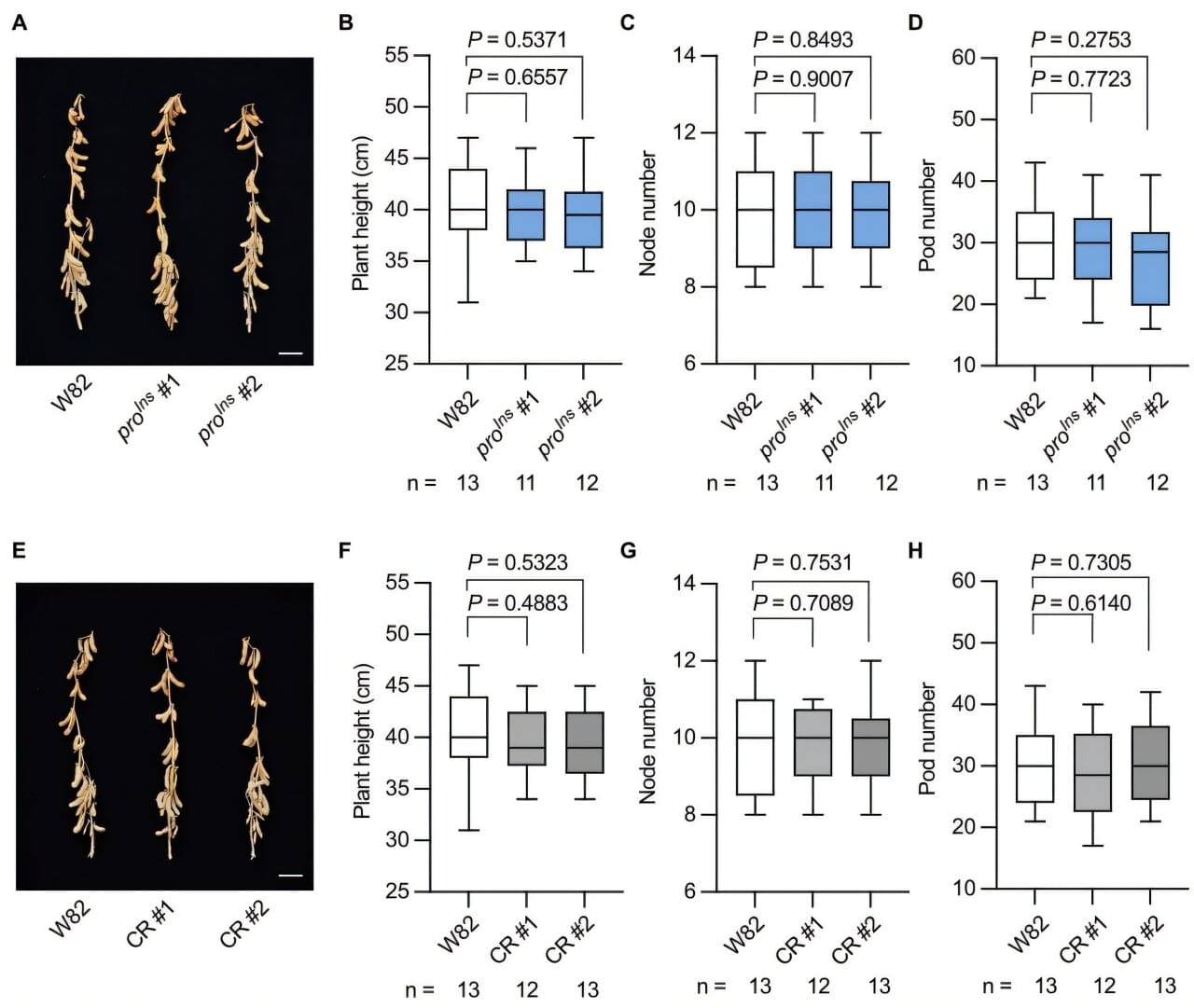
A research team led by Prof. Hou Xingliang from the South China Botanical Garden of the Chinese Academy of Sciences has used genome-wide association studies (GWAS) to identify a rare allele that controls seed protein content and was lost during soybean domestication.
Their findings were published in the Proceedings of the National Academy of Sciences on Oct. 30.
Domesticating wild plants into crops represents a breakthrough in human history, yet key beneficial traits are often lost in the process. Soybeans are a good example. Modern soybean cultivars have lower seed protein content (30%–40%) than their wild ancestors, wild soybeans (Glycine soja), which typically contain 50%–60% protein. Since soybeans (Glycine max [L.] Merr.) are the primary source of plant-based protein for both livestock feed and human nutrition, increasing seed protein content is a critical goal for agricultural research.
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhDDiscount Links/Affiliates: Blood testing (where I get the majority of my labs): https://www.u…

Interval running condenses the powerful effects of regular running into shorter, high-intensity bursts. Research shows it can improve cardiovascular health, regulate blood sugar, and reduce body fat more effectively than longer steady runs. Just a few short sprints per session can deliver major fitness gains.
Running offers a wide range of advantages for both body and mind. It can protect against disease, improve mood, and even slow down the body’s natural aging process.
Yet about 31% of adults still don’t get enough physical activity, including running. The most common reason people give is simple — they don’t have enough time.
Age Reversal in Primates has been achieved. We have it now.
Anti-aging gene therapy, stem cell rejuvenation, and FOXO3 longevity research take center stage in this episode of Longevity Science News with Emmett Short. This groundbreaking study out of Beijing shows that gene-edited human stem cells—specifically FOXO3-enhanced senescence-resistant mesenchymal progenitor cells (SRCs)—can reverse biological aging in elderly monkeys, restoring youthful brain structure, bone density, immune strength, and even ovarian function. By upgrading the FOXO3 longevity gene, scientists created stem cells that resist cellular senescence, DNA damage, and oxidative stress, effectively making the monkeys younger from the inside out. MRI scans revealed increased cortical thickness and improved memory-related connectivity, while biological age clocks showed a 3–5 year reversal across 54% of tissues—equivalent to 9–15 years of human rejuvenation. Emmett explains how these anti-aging stem cells, epigenetic resets, and exosome-based rejuvenation pathways could revolutionize regenerative medicine, longevity biotech, and future human trials. He also explores the costs, ethics, and long-term implications of turning back time at the cellular level. If you’re passionate about biohacking, gene editing, lifespan extension, or the future of anti-aging science, this is the video for you.
HUME BODY ANALYZER:
Use Code: LONGEVITY for up to 50% OFF
https://humehealth.com//discount/LONGEVITY?redirect=/pages/h…=LONGEVITY
Watch next: Artificial Blood: The SciFi Anti-Aging Tech That’s Now in Human Trials.
https://www.youtube.com/watch?v=3xz1lcGdQPc.
If you’re into the cutting edge of anti-aging science, subscribe and share with someone who wants to live longer, healthier, and stronger.
⏱️ Chapters:
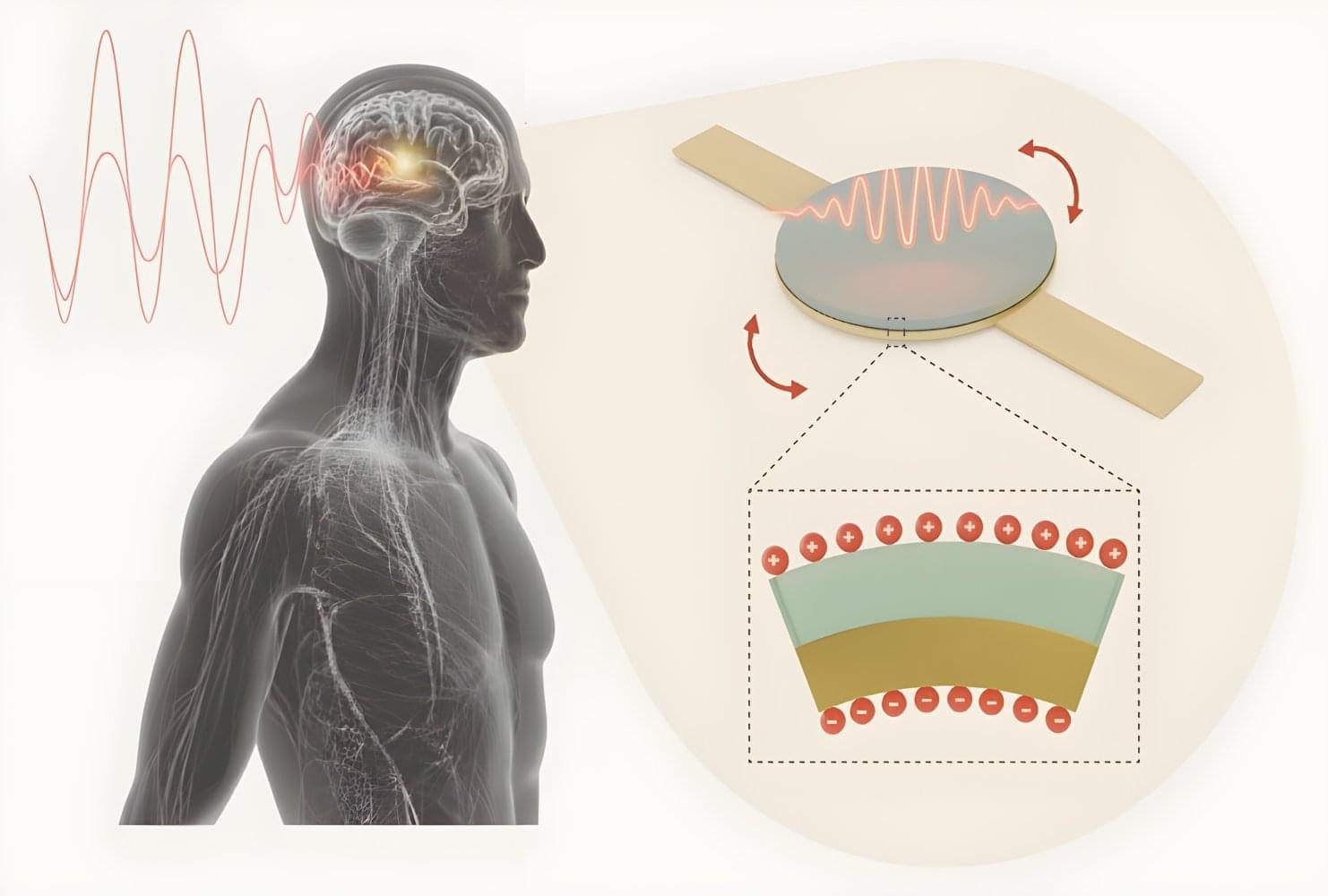
MIT researchers created a needle-injectable, sand-sized magnetoelectric antenna that wirelessly powers deep-tissue implants using low-frequency magnetic fields. The tiny antenna delivers far greater power and safety than conventional antennas, enabling scalable, battery-free, minimally invasive bioelectronic implants.
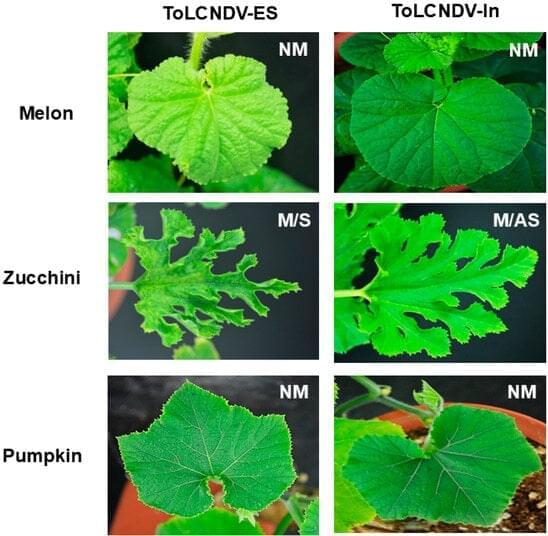
Among begomovirus species, tomato leaf curl New Delhi virus (ToLCNDV) is significant and stands out as a mechanically transmissible bipartite begomovirus originating from the Old World. However, the mechanisms underlying the mechanical transmission of different ToLCNDV strains remain understudied, as their natural transmission occurs via insect vectors. In this study, we investigated the mechanical transmissibility of two ToLCNDVs, one from Italy and another from Pakistan, in host plants. Several cucurbit species were screened, and symptom differences between the two ToLCNDV clones were observed only in zucchini when subjected to rubbing inoculation. The Italian isolate (ToLCNDV-ES) induced typical disease symptoms such as leaf curling, yellow mosaic, and internode stunting, whereas a normal phenotype was observed in zucchini mechanically infected with ToLCNDV-In (Pakistani isolate).