Clinical studies suggest the therapeutic potential of psychedelics, including ayahuasca, DMT, psilocybin, and LSD, in stress-related disorders. These substan…

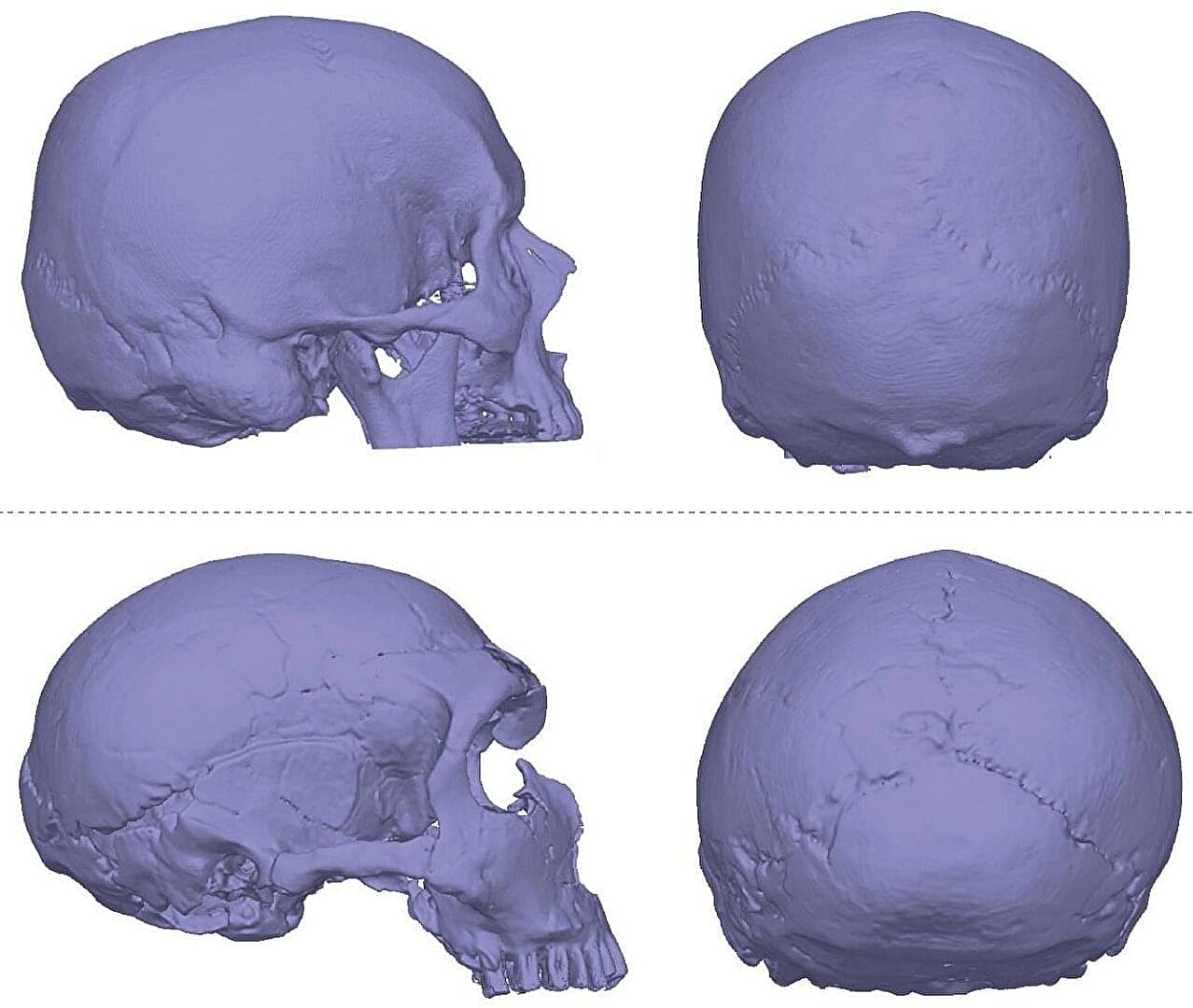
If you regularly experience headaches, dizziness, balance problems and blurred vision, our Neanderthal cousins could be to blame.
These are common symptoms of Chiari malformations, structural defects in which the lower part of the brain extends into the spinal cord. People with this condition have skulls shaped like those of our ancient relatives, leading to a hypothesis (known as the Archaic Homo Introgression Hypothesis) that it may be a genetic legacy from interbreeding between modern humans and Neanderthals.
To investigate this, Kimberly Plomp of the University of the Philippines Diliman and colleagues zeroed in on Chiari 1, the mildest form of the condition, which affects around 1 in 100 people.
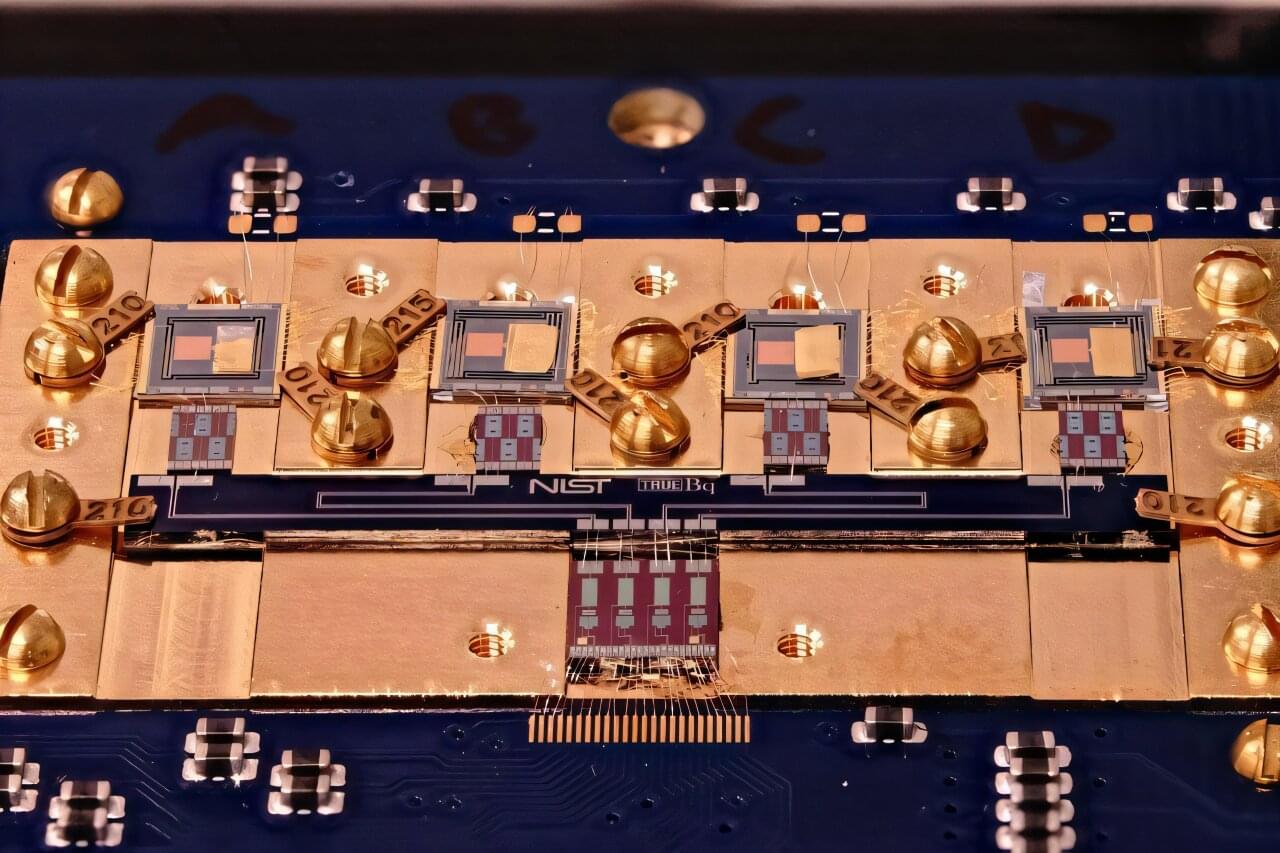
Researchers at the National Institute of Standards and Technology (NIST) have demonstrated a new and faster method for detecting and measuring the radioactivity of minuscule amounts of radioactive material. The innovative technique, known as cryogenic decay energy spectrometry (DES), could have far-reaching impacts, from improving cancer treatments to ensuring the safety of nuclear waste cleanup.
The NIST team has published its results in Metrologia.
The key to this novel technique is a transition-edge sensor (TES), a high-tech device widely used to measure radiation signatures. TES provides a revolutionary capability to record individual radioactive decay events, in which an unstable atom releases one or more particles. By building up data from many individual decays, researchers can then identify which unstable atoms, known as radionuclides, produce the events.
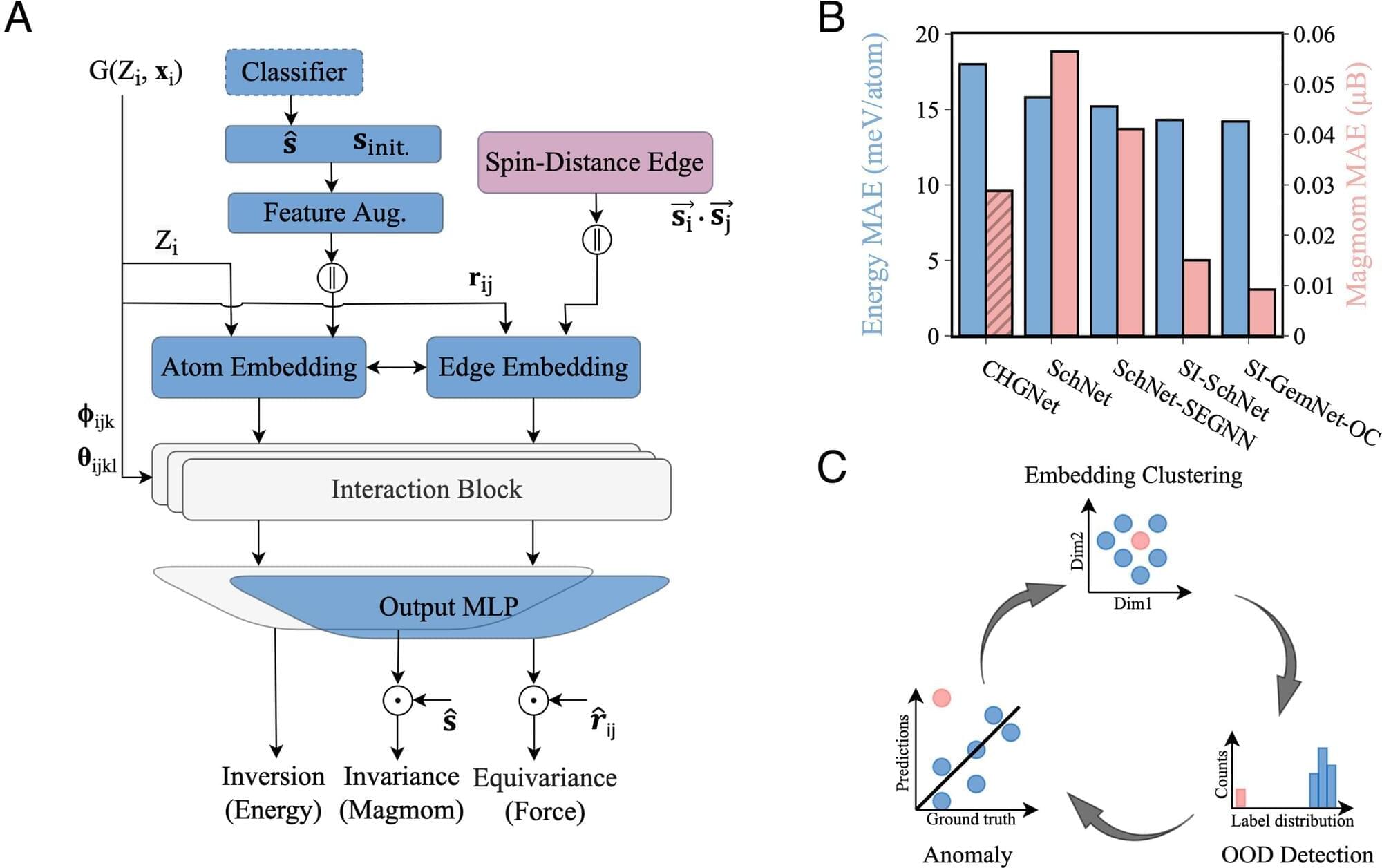
Magnetic materials are in high demand. They’re essential to the energy storage innovations on which electrification depends and to the robotics systems powering automation. They’re also inside more familiar products, from consumer electronics to magnetic resonance imaging (MRI) machines.
Current sources and supply chains won’t be able to keep up as demand continues to grow. We need to design new magnetic materials, and quickly.
A collaboration between Carnegie Mellon University, Lawrence Berkeley National Laboratory, and the Fritz-Haber-Institut der Max-Planck-Gesellschaft is broadening capabilities to screen potential new materials with machine learning models.
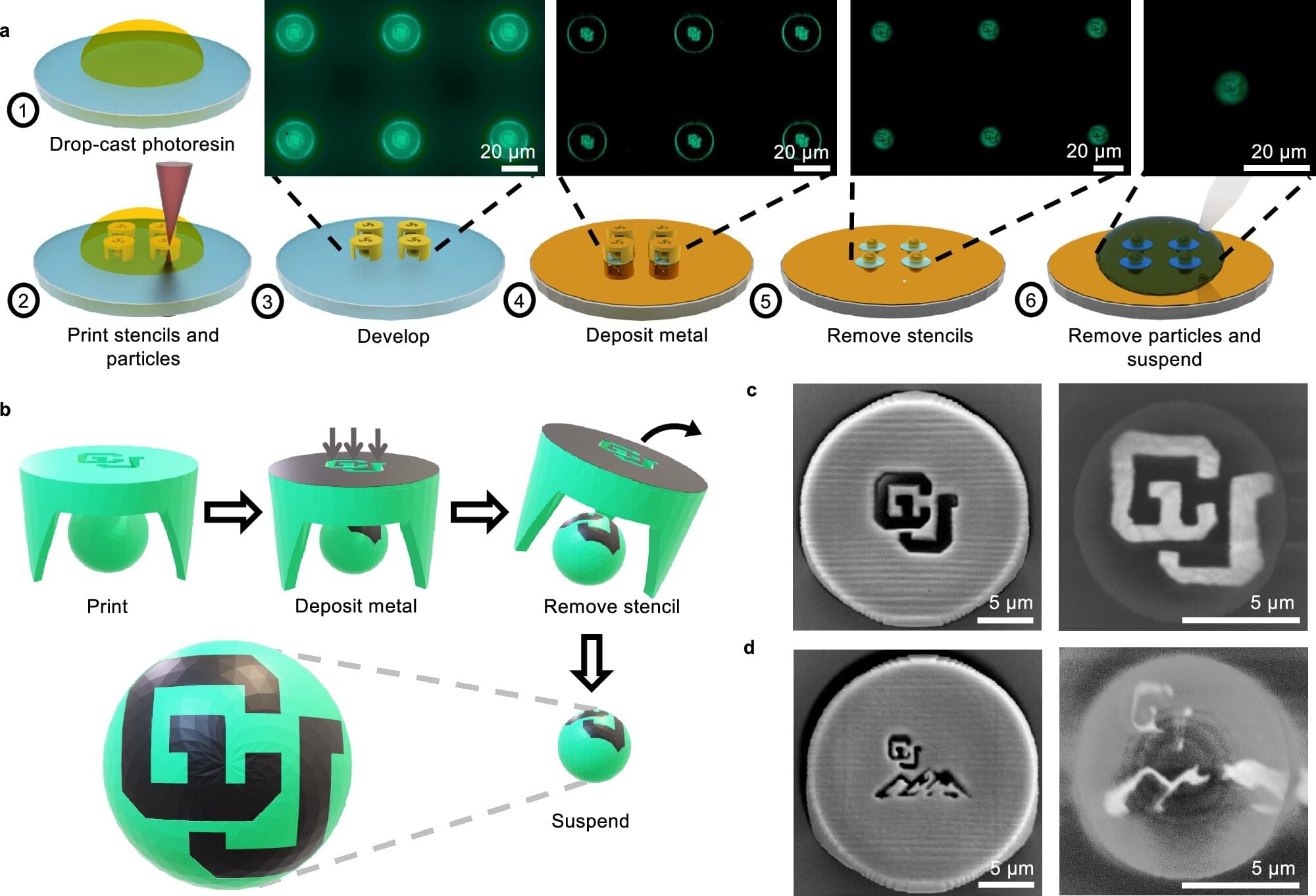
Researchers at the University of Colorado Boulder have created a new way to build and control tiny particles that can move and work like microscopic robots, offering a powerful tool with applications in biomedical and environmental research.
The study, published in Nature Communications, describes a new method of fabrication that combines high-precision 3D printing, called two-photon lithography, with a microstenciling technique. The team prints both the particle and its stencil together, then deposits a thin layer of metal—such as gold, platinum or cobalt—through the stencil’s openings. When the stencil is removed, a metal patch remains on the particle.
The particles, invisible to the naked eye, can be made in almost any shape and patterned with surface patches as small as 0.2 microns—more than 500 times thinner than a human hair. The metal patches guide how the particles move when exposed to electric or magnetic fields, or chemical gradients.
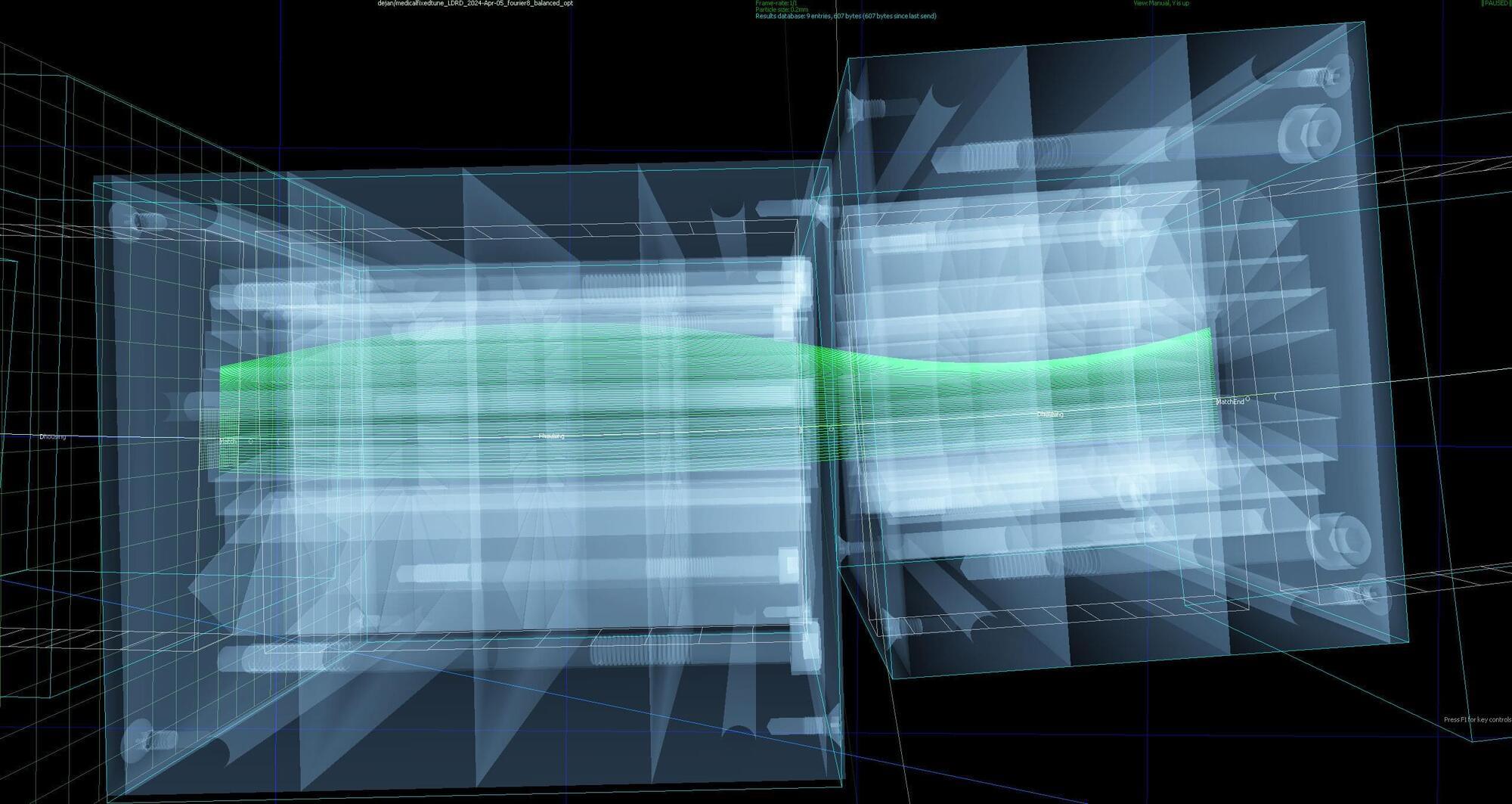
While radiation treatments designed to kill cancer cells have come a long way, scientists and doctors are always exploring new ways to zap tumors more effectively. Recent tests at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory show that a small array of magnets designed as an offshoot of the Lab’s nuclear physics research could quite literally provide a path for such future cancer treatments.
The tests revealed that an arc of meticulously designed permanent magnets can transport beams of cancer-killing protons over a broad range of energies, from 50 to 250 million electron volts (MeV). “That’s the highest energy ever for this sort of beamline,” said Brookhaven Lab physicist Stephen Brooks, designer of the fixed-field magnets, and it’s an energy range that could enable more effective cancer treatment.
Specifically, the project is a step toward a possible future accelerator built using this technology, where physicians could rapidly switch among beam energies to deliver very fast lethal proton doses throughout a tumor’s depth.

In a discovery that could reshape approaches to regenerative medicine and bone repair, researchers have found that human stem cells can be prompted to begin turning into bone cells simply by squeezing through narrow spaces.
The study suggests that the physical act of moving through tight, confining spaces, like those between tissues, can influence how stem cells develop. This could open new possibilities for engineering materials and therapies by guiding cell behavior using physical, rather than chemical, signals.
The research was led by Assistant Professor Andrew Holle from the Department of Biomedical Engineering in the College of Design and Engineering at the National University of Singapore (NUS), and the Mechanobiology Institute (MBI) at NUS, and was published on 8 May 2025 in the journal Advanced Science.
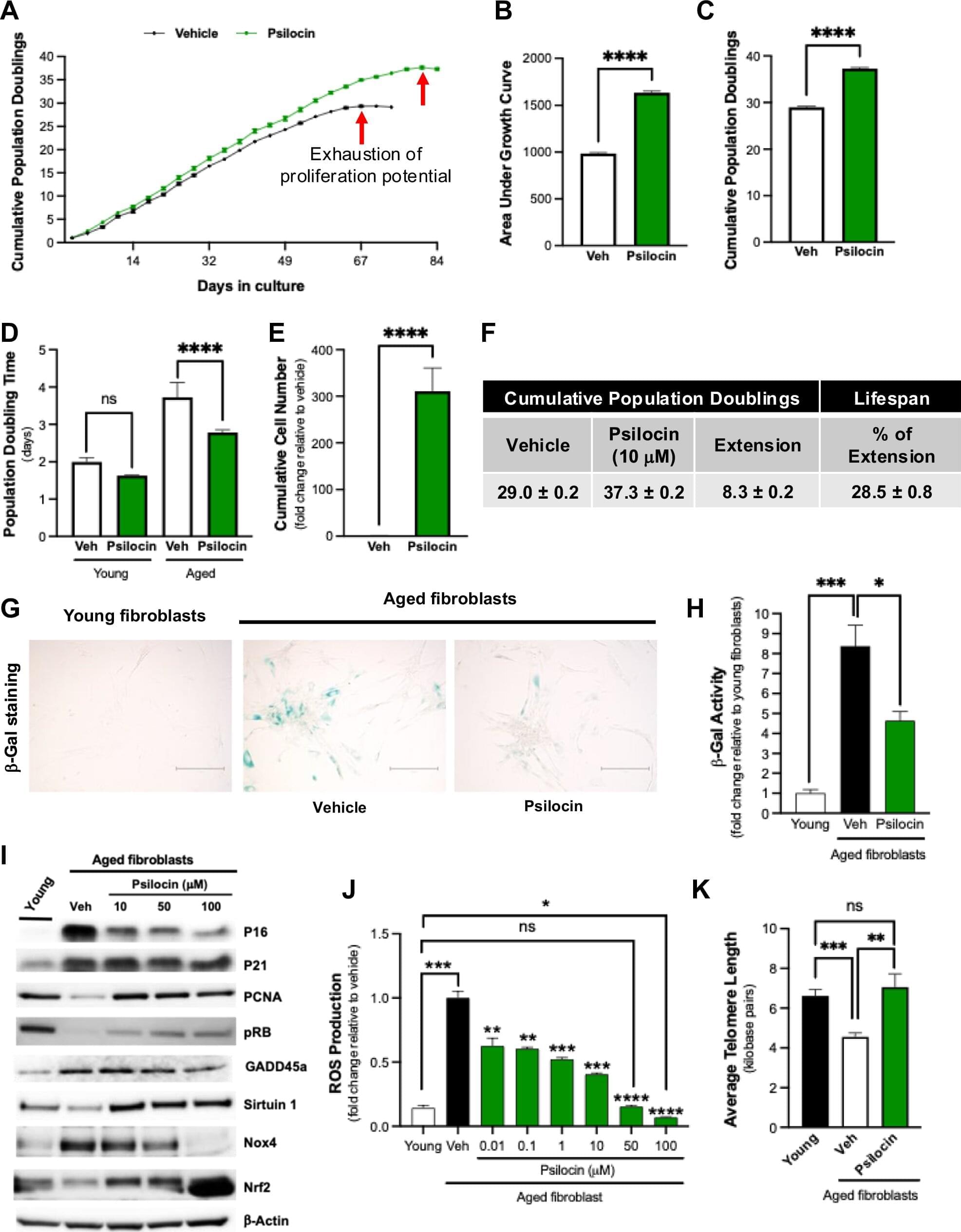
A compound found in psychedelic mushrooms may have antiaging properties. Researchers at Baylor College of Medicine have found that psilocybin, the active compound in psychedelic mushrooms, may extend both cellular and organismal lifespans.
The findings, published in the journal npj Aging, show that psilocybin reduced multiple hallmarks of aging in cells while also improving survival in aged mice.
“There have been a number of clinical studies that have explored the therapeutic potential of psilocybin in psychiatric conditions such as depression and anxiety; however, few studies have evaluated its impacts outside the brain,” said Dr. Louise Hecker, associate professor of medicine— cardiovascular research at Baylor and senior author of the study.

A new large-scale study spotlights postoperative delirium as a preventable and high-impact complication which is driven by patient frailty and surgical stress—and one that can be addressed through low-cost, evidence-based interventions.
The findings, which appear in JAMA Network Open, provide a call to action for clinicians, health systems, patients, and families to prioritize brain health throughout perioperative care.
“Postoperative delirium isn’t a minor complication—it’s analogous to acute brain failure, a medical emergency that should be recognized and addressed,” said Laurent Glance, MD, a professor of Anesthesiology and Perioperative Medicine at the University of Rochester Medical Center (URMC) and senior author of the study.
