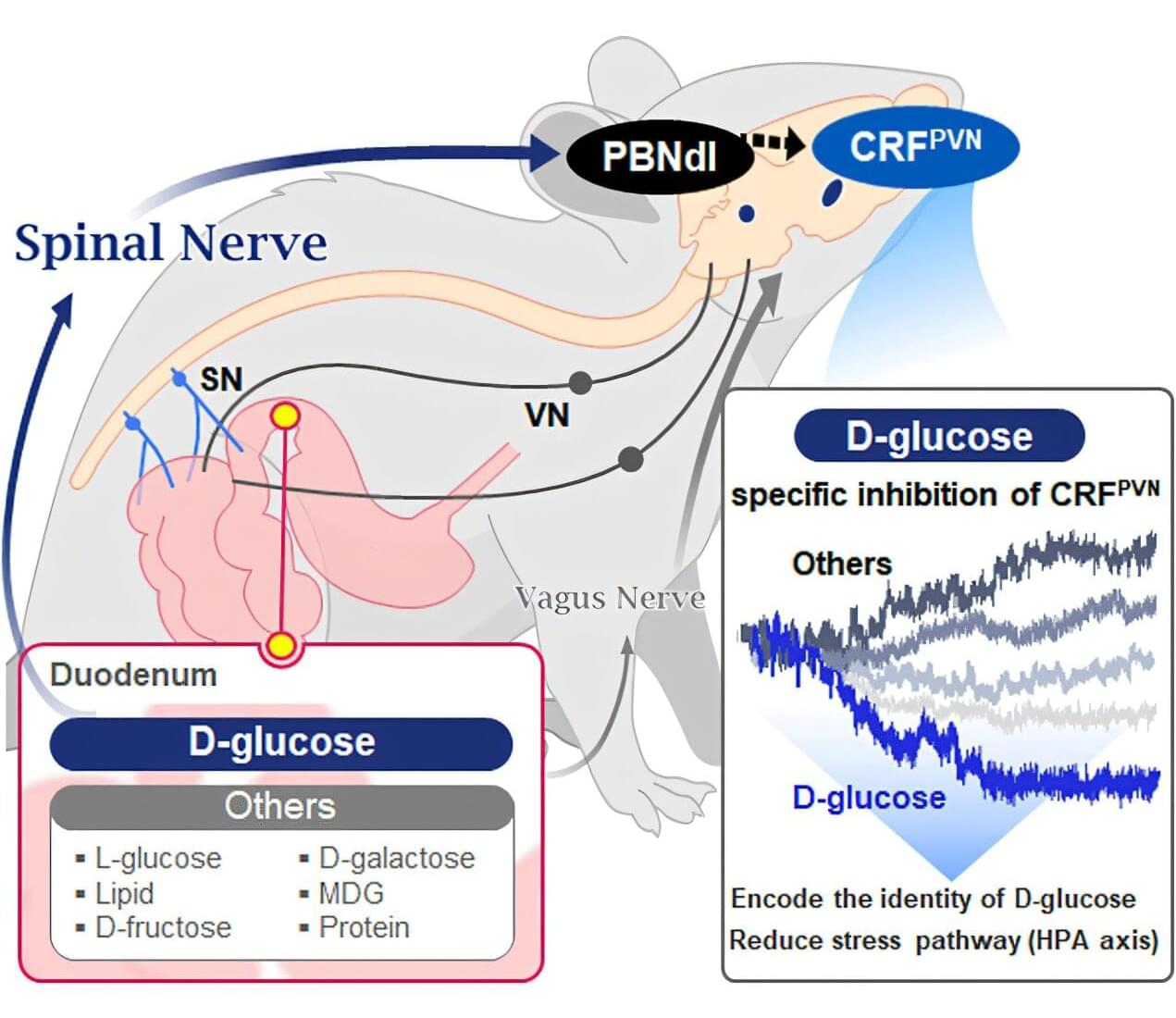
Starting with the question “How does our brain distinguish glucose from the many nutrients absorbed in the gut?” a KAIST research team has demonstrated that the brain can selectively recognize specific nutrients—particularly glucose—beyond simply detecting total calorie content. Their study, published in Neuron, is expected to offer a new paradigm for appetite control and the treatment of metabolic diseases.
Professor Greg S.B. Suh’s team in the Department of Biological Sciences, in collaboration with Professor Young-Gyun Park’s team (BarNeuro), Professor Seung-Hee Lee’s team (Department of Biological Sciences), and the Albert Einstein College of Medicine in New York, have identified the existence of a gut– brain circuit that allows animals in a hungry state to selectively detect and prefer glucose in the gut.
Organisms derive energy from various nutrients, including sugars, proteins, and fats. Previous studies have shown that total caloric information in the gut suppresses hunger neurons in the hypothalamus to regulate appetite. However, the existence of a gut–brain circuit that specifically responds to glucose and corresponding brain cells had not been demonstrated until now.