Simple calculations, such as factoring low numbers, can be made by mixing together differently shaped strands of DNA


Simple calculations, such as factoring low numbers, can be made by mixing together differently shaped strands of DNA
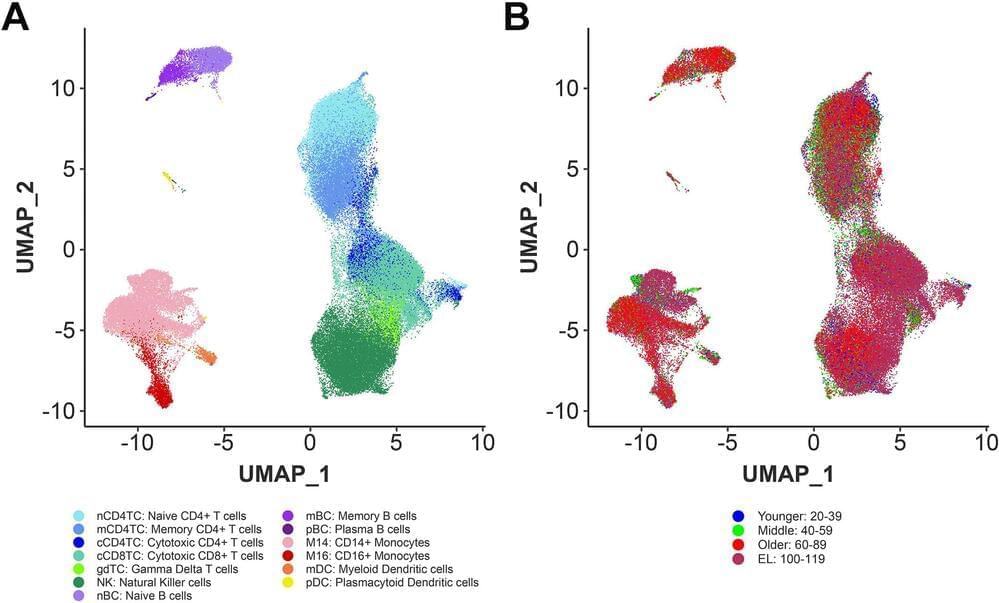
There are approximately 30 trillion cells in a human body and our health is predicated on them properly interacting with and supporting each other, with the immune system playing a particularly pivotal role. One of the defining characteristics of aging is a decline in the proper functioning of our immune system. Centenarians, a rare population of individuals who reach 100 years or more, experience delays in aging-related diseases and mortality which suggests their immune systems remain functional into extreme old age.
Led by researchers from Boston University Chobanian & Avedisian School of Medicine and Tufts Medical Center, a new study finds centenarians harbor distinct immune cell type composition and activity and possess highly functional immune systems that have successfully adapted to a history of sickness allowing for exceptional longevity. These immune cells may help identify important mechanisms to recover from disease and promote longevity.
“Our data support the hypothesis that centenarians have protective factors that enable to recover from disease and reach extreme old ages,” said lead author Tanya Karagiannis, Ph.D., senior bioinformatician, Center for Quantitative Methods and Data Science, Institute for Clinical Research and Health Policy Studies at Tufts Medical Center.

According to statistical analysis, Japanese women, in particular, may live to be 122 in the coming decades.
Whether or not there is a limit to the human lifespan has been a subject of debate for millennia. However, estimates indicate that the maximum lifespan has increased throughout recorded history. For instance, the late Bronze Age’s Hebrews regarded 80 years as the maximum human length, then 1,000 years later, the Romans considered it to be 100 years.
Skiping to the present, Jeanne Calment, who died in 1997 at the age of 122, currently holds the world record for the oldest person. Despite advances in medical science, no one has been able to break this record so far.
Cecilie_Arcurs/iStock.
However, estimates indicate that the maximum lifespan has increased throughout recorded history. For instance, the late Bronze Age’s Hebrews regarded 80 years as the maximum human length, then 1,000 years later, the Romans considered it to be 100 years.
Engineers at MIT and the University of Massachusetts Medical School have designed a new type of nanoparticle that can be administered to the lungs, where it can deliver messenger RNA encoding useful proteins.
With further development, these particles could offer an inhalable treatment for cystic fibrosis and other diseases of the lung, the researchers say.
“This is the first demonstration of highly efficient delivery of RNA to the lungs in mice. We are hopeful that it can be used to treat or repair a range of genetic diseases, including cystic fibrosis,” says Daniel Anderson, a professor in MIT’s Department of Chemical Engineering and a member of MIT’s Koch Institute for Integrative Cancer Research and Institute for Medical Engineering and Science (IMES).

Turning genes on and off as easily and predictably as flicking a switch could be a powerful tool in medicine and biotech. A type of technology called a riboswitch might be the key. The Okinawa Institute of Science and Technology (OIST) in Japan, in collaboration with Astellas Pharma Inc., has developed a new toolkit that uses small molecules to control the activity of a piece of synthetic RNA, and ultimately regulate gene expression. The technology, which was described in the Journal of the American Chemical Society, worked in mammalian cell cultures and in mice.
The ability to precisely control whether a gene is turned on or off is expected to lead to more efficient production of compounds that are made using animal cells, and make gene therapy, cell therapy, and regenerative medicine safer.
For genes to be expressed, cells make many RNA copies of a section of DNA. These RNA copies, called transcripts, are then used to make the protein. This can lead to the introduction of additional genes (either as DNA or RNA) into cells, which can then be used to make new proteins for a wide variety of applications.
Why do cancer immunotherapies work so extraordinarily well in a minority of patients, but fail in so many others? By analyzing the role of neutrophils, immune cells whose presence usually signals treatment failure, scientists from the University of Geneva (UNIGE), from Harvard Medical School, and from Ludwig Cancer Center have discovered that there is not just one type of neutrophil, but several. Depending on certain markers on their surface, these cells can either promote the growth of tumors, or fight them and ensure the success of a treatment. By boosting the appropriate factors, neutrophils could become great agents of anti-tumor immunity and reinforce the effects of current immunotherapies. These results have been published in the journal Cell.
Immunotherapy involves activating immune cells —mainly T cells—to recognize and destroy cancer cells. While this treatment is very efficient for some patients, and sometimes even exceeds expectations, it is unfortunately not the case in most cases. “The reasons for these failures remain largely unknown,” says Mikaël Pittet, full professor at the UNIGE Faculty of Medicine, holder of the ISREC chair in immuno-oncology, director of the Centre for Translational Research in Onco-Hematology and member of the Ludwig Cancer Center, who directed this work. “This is why deciphering the immune components involved is key to develop more advanced treatments and make immunotherapies a real therapeutic revolution.”
Neutrophils are the most abundant immune cells in the blood and are very useful in infections or injuries by being quickly mobilized to the affected area and releasing antimicrobial factors. In the context of cancer, however, their presence is generally bad news as they promote vascularization and tumor progression.
In a recent study on the medRxiv preprint server, researchers identified an interleukin 6 (IL-6) dependent alternative pathway as a therapeutic strategy against coronavirus disease 2019 (COVID-19).
Study: A Complement Atlas identifies interleukin 6 dependent alternative pathway dysregulation as a key druggable feature of COVID-19. Image Credit: MarynaOlyak/Shutterstock.com

The expanded availability of opioid use disorder-related telehealth services and medications during the COVID-19 pandemic was associated with a lowered likelihood of fatal drug overdose among Medicare beneficiaries, according to a new study.
“The results of this study add to the growing research documenting the benefits of expanding the use of telehealth services for people with opioid use disorder, as well as the need to improve retention and access to medication treatment for opioid use disorder,” said lead author Christopher M. Jones, PharmD, DrPH, director of the National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. “The findings from this collaborative study also highlight the importance of working across agencies to identify successful strategies to address and get ahead of the constantly evolving overdose crisis.”
Published today in JAMA Psychiatry, this study is a collaborative research effort led by researchers at the National Center for Injury Prevention and Control, a part of CDC; the Office of the Administrator and the Center for Clinical Standards and Quality, both part of the Centers for Medicare & Medicaid Services (CMS); and the National Institute on Drug Abuse, a part of the National Institutes of Health.
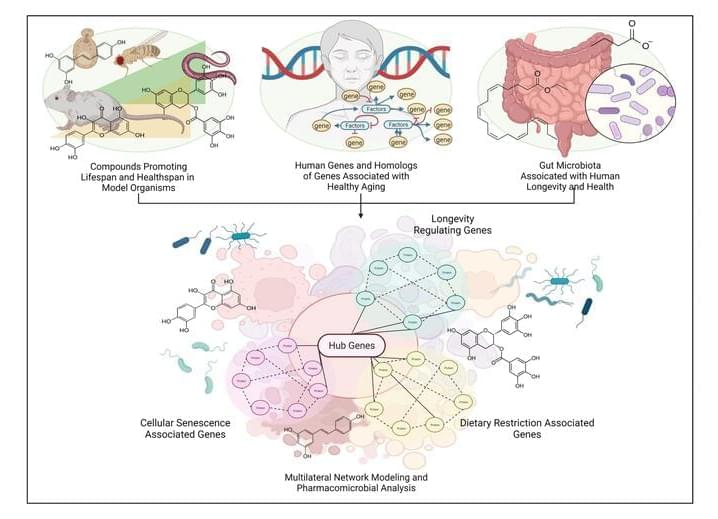
Advances in antiaging drug/lead discovery in animal models constitute a large body of literature on novel senotherapeutics and geroprotectives. However, with little direct evidence or mechanism of action in humans—these drugs are utilized as nutraceuticals or repurposed supplements without proper testing directions, appropriate biomarkers, or consistent in-vivo models. In this study, we take previously identified drug candidates that have significant evidence of prolonging lifespan and promoting healthy aging in model organisms, and simulate them in human metabolic interactome networks. Screening for drug-likeness, toxicity, and KEGG network correlation scores, we generated a library of 285 safe and bioavailable compounds. We interrogated this library to present computational modeling-derived estimations of a tripartite interaction map of animal geroprotective compounds in the human molecular interactome extracted from longevity, senescence, and dietary restriction-associated genes. Our findings reflect previous studies in aging-associated metabolic disorders, and predict 25 best-connected drug interactors including Resveratrol, EGCG, Metformin, Trichostatin A, Caffeic Acid and Quercetin as direct modulators of lifespan and healthspan-associated pathways. We further clustered these compounds and the functionally enriched subnetworks therewith to identify longevity-exclusive, senescence-exclusive, pseudo-omniregulators and omniregulators within the set of interactome hub genes. Additionally, serum markers for drug-interactions, and interactions with potentially geroprotective gut microbial species distinguish the current study and present a holistic depiction of optimum gut microbial alteration by candidate drugs. These findings provide a systems level model of animal life-extending therapeutics in human systems, and act as precursors for expediting the ongoing global effort to find effective antiaging pharmacological interventions.
Communicated by Ramaswamy H. Sarma.