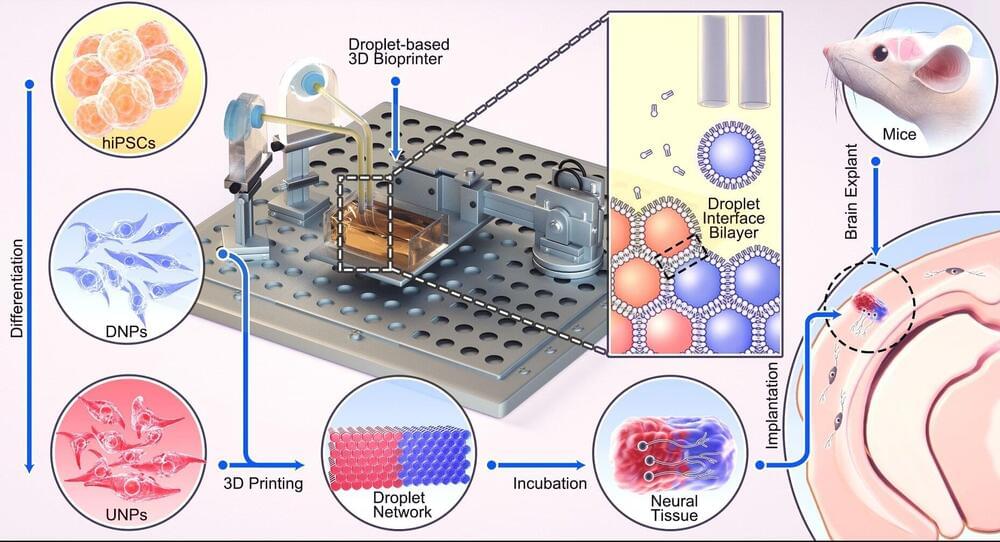
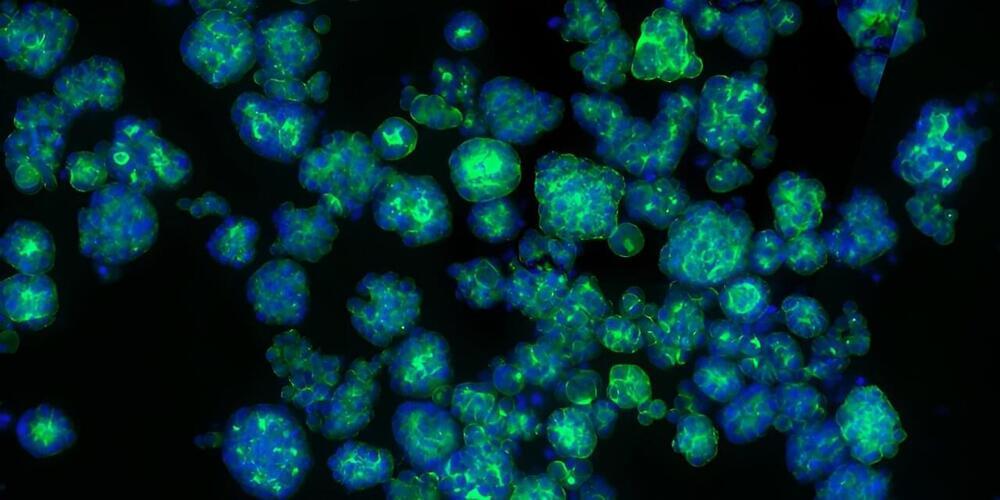
A breakthrough technique developed by University of Oxford researchers could one day provide tailored repairs for those who suffer brain injuries. The researchers have demonstrated for the first time that neural cells can be 3D-printed to mimic the architecture of the cerebral cortex. The results have been published in the journal Nature Communications.
Brain injuries, including those caused by trauma, stroke, and surgery for brain tumors, typically result in significant damage to the cerebral cortex (the outer layer of the human brain), leading to difficulties in cognition, movement and communication. For example, each year, around 70 million people globally suffer from traumatic brain injury (TBI), with 5 million of these cases being severe or fatal. Currently, there are no effective treatments for severe brain injuries, leading to serious impacts on quality of life.
Tissue regenerative therapies, especially those in which patients are given implants derived from their own stem cells, could be a promising route to treat brain injuries in the future. Up to now, however, there has been no method to ensure that implanted stem cells mimic the architecture of the brain.