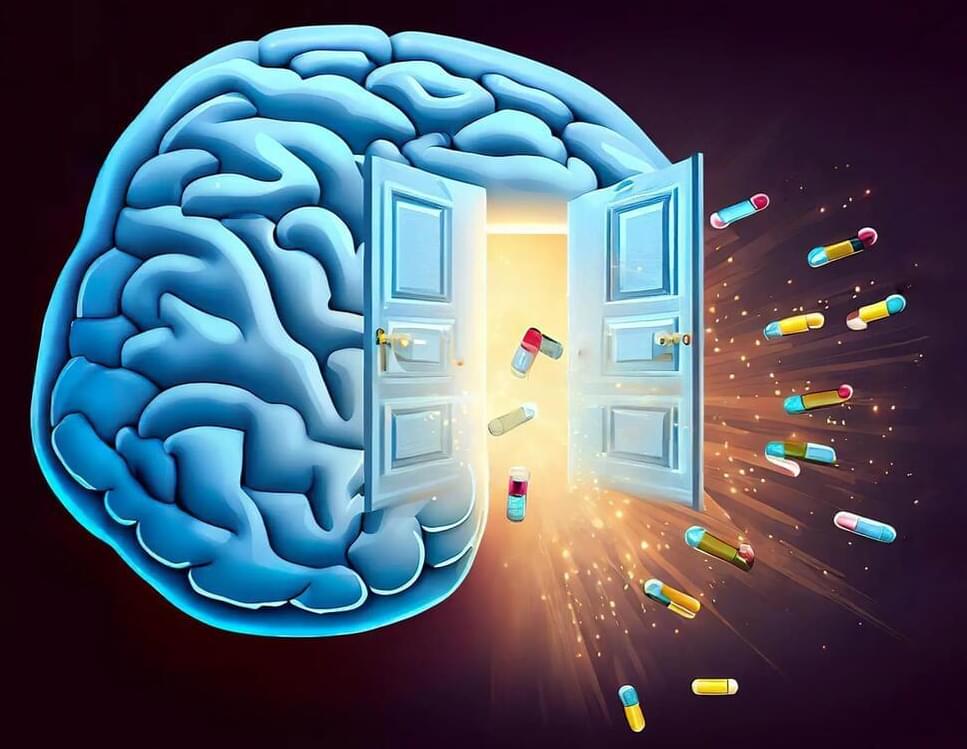
Caltech researchers discovered an enzyme that enables viral vectors to cross the blood-brain barrier, potentially aiding brain disorder drug development and research.
The blood–brain barrier (BBB) is a stringent, nearly impenetrable layer of cells that guards the brain, protecting the vital organ from hazards in the bloodstream such as toxins or bacteria and allowing only a very limited set of small molecules, such as nutrients, to pass through. This layer of protection, however, makes it difficult for researchers to study the brain and to design drugs that can treat brain disorders.
Now, a new study from Caltech has identified a previously unknown mechanism by which certain viral vectors—protein shells engineered to carry various desired cargo—can cross through the BBB. This mechanistic insight may provide a new approach to designing viral vectors for research and therapeutic applications. Understanding this and other new mechanisms could also give insight into how the brain’s defenses may be exploited by emergent pathogens, enabling researchers to prepare methods to block them.