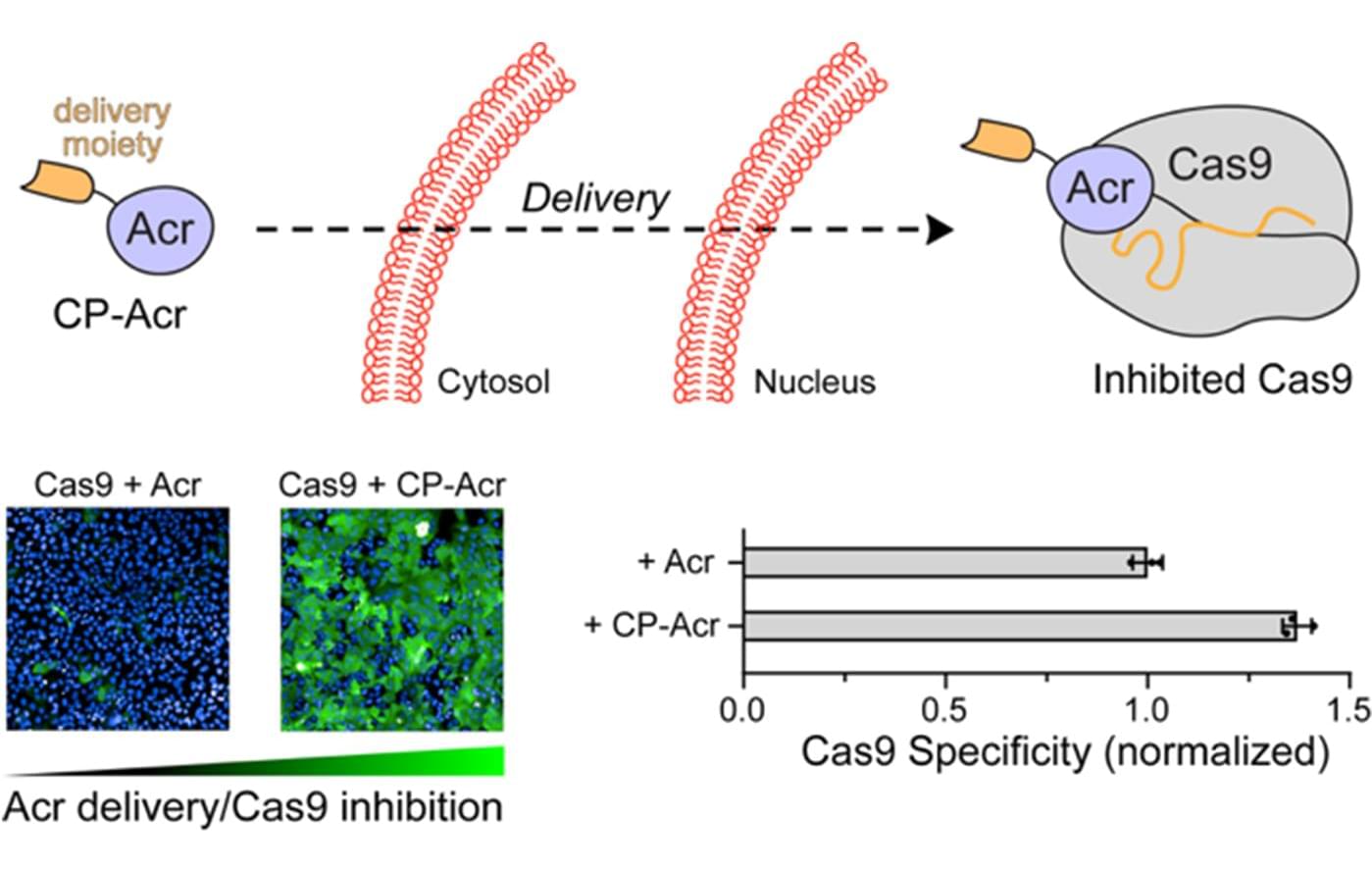
One of the most exciting developments in cancer treatment is a wave of new cell therapies that train a patient’s immune system to attack cancer cells. Such therapies have saved the lives of patients with certain aggressive cancers and few other options. Most of these therapies work by teaching immune cells to recognize and attack specific proteins on the surface of cancer cells.
Unfortunately, most proteins found on cancer cells aren’t unique to tumors. They’re also often present on healthy cells, making it difficult to target cancer aggressively without triggering dangerous attacks on other tissue. The problem has limited the application of cell therapies to a small subset of cancers.
Now Senti Bio is working to create smarter cell therapies using synthetic biology. The company, which was founded by former MIT faculty member and current MIT Research Associate Tim Lu ’03, MEng ’03, PhD ’08 and Professor James Collins, is equipping cells with gene circuits that allow the cells to sense and respond to their environments.
Founded by MIT researchers, Senti Bio is is working to create smarter cell therapies for cancer using synthetic biology. The company equips cells with gene circuits that allow the cells to respond to their environments.