In this Review, Bengel and colleagues propose a roadmap for the clinical implementation of radiotracer-based molecular imaging of immune and fibrotic pathways to guide targeted therapies for heart repair after myocardial infarction.


Using advanced computer simulations, researchers from the University of Rhode Island’s Graduate School of Oceanography (GSO) have concluded how and why strong ocean currents modify surface waves. “Our primary finding is that hurricane-generated ocean currents can substantially reduce both the height and the dominant period of hurricane waves,” said Isaac Ginis, URI professor of oceanography. “The magnitude of wave reduction depends strongly on how accurately ocean currents are predicted. This highlights the importance of using fully coupled wave-ocean models when forecasting hurricane waves.”
Ginis conducted the research with URI Professor Tetsu Hara and Angelos Papandreou, who earned his Ph.D. in oceanography from URI in December 2025. Their results were published in a peer-reviewed article in the Journal of Physical Oceanography in January 2026.
According to Ginis, waves are most strongly reduced by currents on the front right of the storm, where winds, waves, and currents are typically strongest.

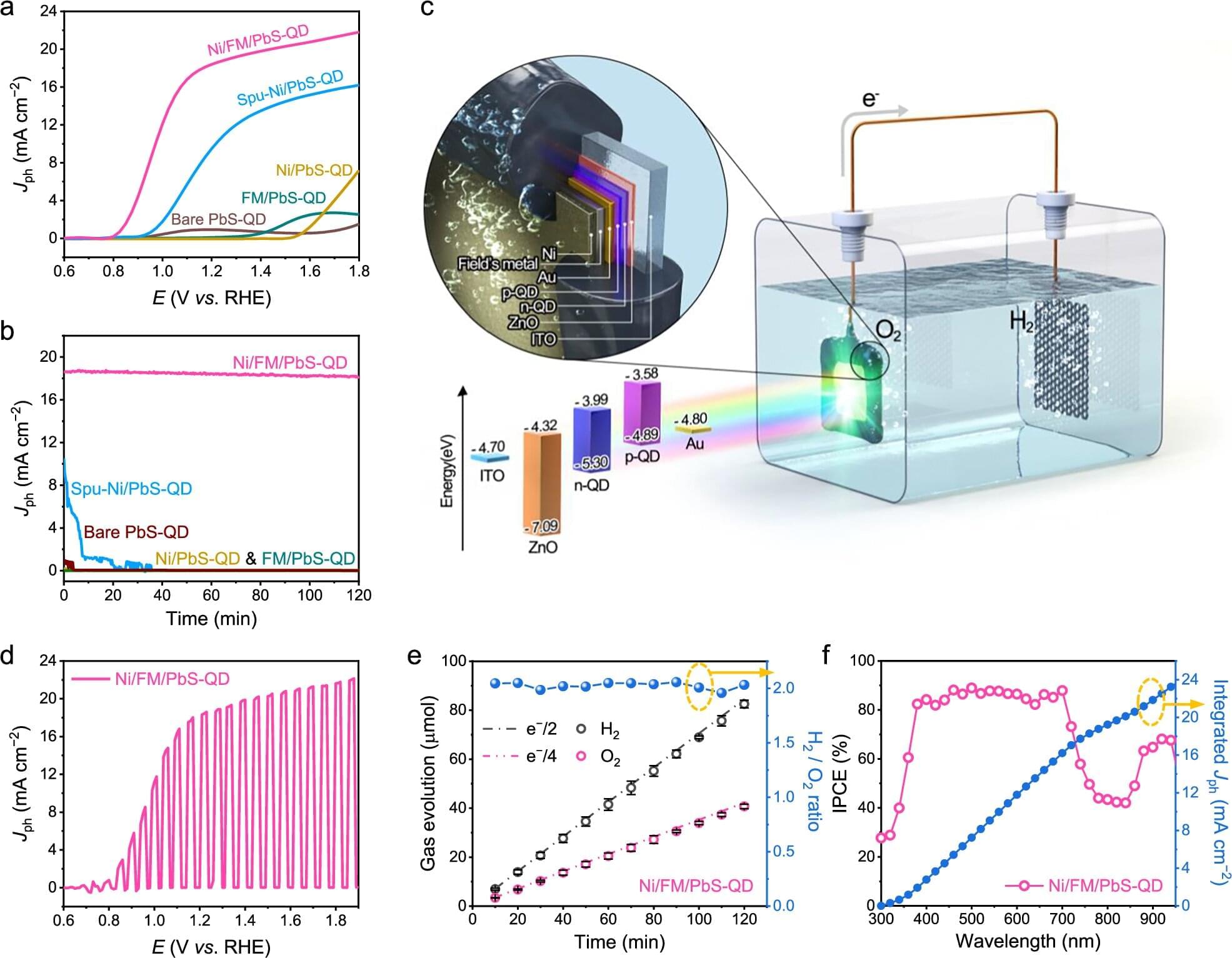
A research team affiliated with UNIST has developed stable and efficient chalcogenide-based photoelectrodes, addressing a longstanding challenge of corrosion. This advancement paves the way for the commercial viability of solar-driven water splitting technology—producing hydrogen directly from sunlight without electrical input.
Jointly led by Professors Ji-Wook Jang and Sung-Yeon Jang from the School of Energy and Chemical Engineering, the team reported a highly durable, corrosion-resistant metal-encapsulated PbS quantum dot (PbS-QD) solar cell-based photoelectrode that delivers both high photocurrent and long-term operational stability for photoelectrochemical (PEC) water splitting without the need for sacrificial agents. The research is published in the journal Nature Communications.
PEC water splitting is a promising route for sustainable hydrogen production, where sunlight is used to drive the decomposition of water into hydrogen and oxygen within an electrolyte solution. The efficiency of this process depends heavily on the stability of the semiconductor material in the photoelectrode, which absorbs sunlight and facilitates the electrochemical reactions. Although chalcogenide-based sulfides, like PbS are highly valued for their excellent light absorption and charge transport properties, they are prone to oxidation and degradation when submerged in water, limiting their operational stability.

In healthy aging strategies, nutritional supplements synergize with optimized dietary and lifestyle interventions by modulating aging-related molecular pathways.[ 8, 9 ] Notably, NMN exerts multi-organ anti-aging effects by elevating NAD+ levels to activate the SIRT1 pathway, thereby significantly enhancing mitochondrial function while reducing oxidative stress and DNA damage.[ 10 ] Similarly, curcumin delays aging and related diseases through pleiotropic mechanisms involving oxidative stress regulation, anti-inflammatory actions, telomere maintenance, and sirtuin protein modulation.[ 11 ] However, practical applications face significant challenges: bioactive compounds like resveratrol and curcumin suffer from limited bioavailability due to poor aqueous solubility and first-pass metabolism, while excessive supplementation of antioxidants such as vitamins C/E may disrupt reactive oxygen species (ROS) signaling homeostasis, potentially inducing cellular toxicity or even increasing hemorrhagic risk.[ 12-14 ] Future development of anti-aging supplements should focus on: 1) innovative formulation strategies to enhance bioavailability; 2) optimized dosing regimens to minimize toxicity; and 3) long-term clinical studies to validate efficacy.
Selenium, an essential trace element with diverse biological activities, plays a critical role in healthy aging.[ 15-17 ] ≈1 billion people worldwide are affected by selenium deficiency, which is closely linked to neurological disorders, cardiovascular abnormalities, malignancies, and immune dysfunction.[ 18-20 ] Substantial evidence supports the anti-aging effects of selenium through multiple mechanisms: 1) Selenomethionine (SeMet) effectively suppresses Fe2+/H2O2- or Aβ-induced free radical generation, demonstrating therapeutic potential for Alzheimer’s disease characterized by oxidative stress;[ 21 ] 2) Selenium supplementation elevates serum GPx3 levels, a selenoprotein predominantly localized in the basement membrane of renal proximal tubules, modulating mitochondrial quality control pathways to mitigate heavy metal-induced renal aging;[ 22 ] and 3) Our recent findings reveal that selenium supplementation significantly attenuates age-related muscle atrophy by preserving redox homeostasis and regulating muscle protein metabolism.[ 23 ] Recent clinical trials in patients with advanced non-small cell lung cancer (NSCLC) demonstrated that oral administration of selenium nanoparticles (SeNPs) as a dietary supplement (200 µg day−1) in combination with Bev+AP chemotherapy significantly enhanced therapeutic outcomes compared to chemotherapy alone. The SeNPs combination group showed remarkable tumor regression, with progression disease rates decreasing dramatically from 50% to 0% and partial response rates increasing to 83.3%, along with significantly improved objective response rate and disease control rate.[ 24 ] Importantly, this regimen maintained excellent safety profiles without triggering fluctuations in pro-inflammatory or immunosuppressive cytokines. These compelling findings not only establish SeNPs as a safe and effective adjuvant therapy for advanced NSCLC but also provide valuable clinical translation data for nano-selenium formulations in oncology. Despite selenium’s proven benefits in reducing oxidative damage, maintaining genomic stability, and delaying telomere shortening, its narrow therapeutic window, limited bioavailability, and specific mechanisms in multi-organ protection during natural aging require further investigation.
Nanodelivery carriers have emerged as a next-generation platform for gene and drug delivery, offering tunable physicochemical properties such as size, composition, and surface modifications.[ 25 ] Our team has developed organically-bridged mesoporous silica nanoparticles (MSNs) by incorporating functional diselenide bonds into the silica framework at the molecular level, addressing the critical challenge of poor biodegradability in conventional silica materials.[ 26 ] This nanocarrier exhibits unique dual redox-responsive properties, allowing for more precise maintenance of redox homeostasis compared to traditional antioxidants, aligning with the core goal of preserving organismal homeostasis in anti-aging research. Building on this breakthrough, a comprehensive research framework was established: first, this study constructed a natural aging mouse model with MSNs, disulfide-bridged MSNs (SMSNs), commercially available SeMet as controls and then compared the effects of diselenide-bridged MSNs (SeMSNs) on lifespan extension, frailty delay, and multi-organ anti-aging. Next, key pathways and targets were identified through multi-organ transcriptome sequencing, followed by in-depth mechanistic studies. Finally, clinical translation was integrated by analyzing the correlation between serum selenium levels and aging biomarkers in the elderly, and validating the clinical effects of SeMSNs using primary adipose precursor cells (APCs) models. This systematic approach provides a solid theoretical foundation and clinical evidence for the application of nano-selenium in anti-aging research.

A letter by distinguished scientists directed at Integrated Information Theory sought to discredit a leading theory of consciousness as pseudoscience. That, argues Erik Hoel, was a mistake.
Hoel contends that as no theory of consciousness is currently empirically testable, it’s impossible for any of these theories to be scientific.
Hoel is a neuroscientist, neurophilosopher, and fiction writer. He’s been a close collaborator of Giulio Tononi, and a Forbes 30 under 30 in science.
“Consciousness, according to IIT, might be more widespread than we think, but it is neither universal nor arbitrary,” writes Hoel.
Tap the link to read more now.


Wnt–NAD+ axis in stem cell function.
The Wnt–NAD+ axis is a fundamental regulatory hub in which metabolic state meets developmental signaling and it acts as a metabolic sensor that coordinates tissue regeneration with cellular energy status through compartment specific NAD+ pools.
Wnt signaling regulates NAD+ metabolism by controlling the expression of key biosynthetic enzymes and NAD+ consumers, while NAD+-dependent proteins modulate Wnt activity through direct interactions and epigenetic modifications.
Sirtuins exhibit tissue-specific and subcellular compartment-dependent roles in Wnt regulation where they function as activators or suppressors depending on the cellular bioenergetic state.
The Wnt–NAD+ axis maintains stem cell function and self-renewal capacity through metabolic/signaling integration, and its disruption during aging leads to declining regenerative capacity.
The progressive dysregulation of compartment-specific Wnt–NAD+ coordination contributes to stem cell exhaustion and multiple pathological conditions, indicating that therapeutic strategies must consider tissue-specific and subcellular targeting. sciencenewshighlights ScienceMission https://sciencemission.com/Wnt%E2%80%93NAD-axis

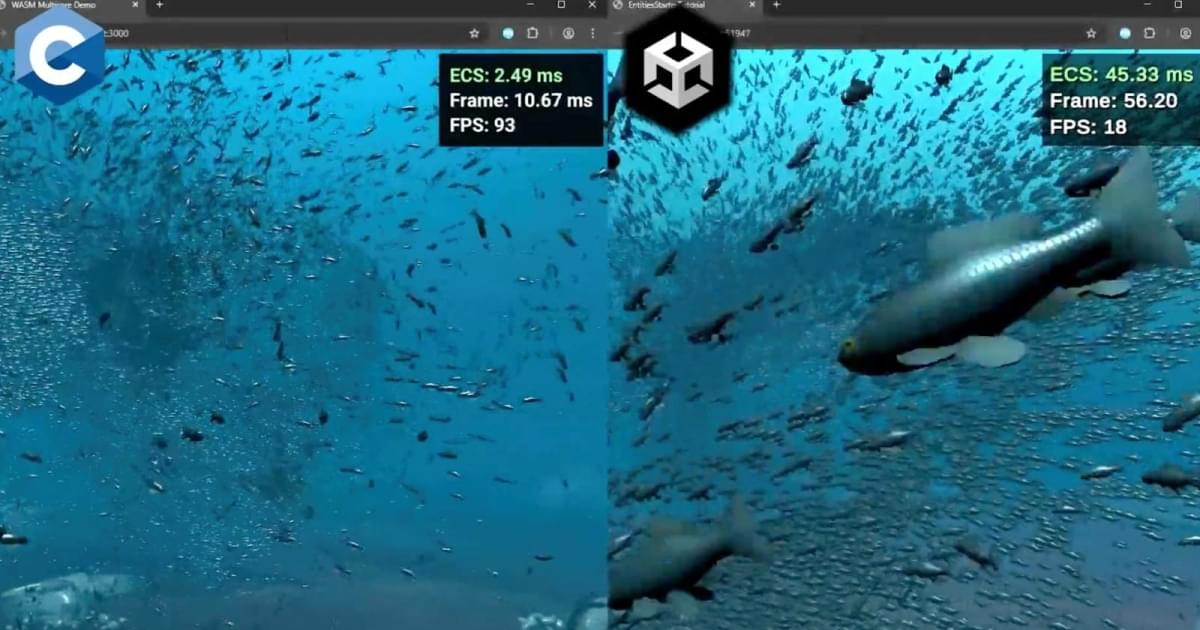
Gabriel Dechichi, a developer you might remember from his challenge of making an Unreal Engine game in 4 weeks, has demonstrated his ECS system in C, claiming it runs roughly 17 times faster than Unity’s DOTS.
According to Gabriel, the simulation runs 100,000 boids, rendering around 31 million triangles per frame. The average simulation time is about 2.4 ms in the C engine, compared to around 44.4 ms in Unity’s ECS. The test was conducted on an AMD Ryzen 7 5800H (8 cores, 16 threads) with an NVIDIA GeForce RTX 3,060 Laptop GPU and 32 GB of RAM, running in Chrome with force-high-performance-GPU enabled.
“Frame time difference is about 5 times, as the demo is GPU-bound. The ECS simulation runs at roughly 2.4ms on my C engine, vs roughly 44.4 ms for Unity ECS. Time is measured equally on both demos by sampling how long the ECS world takes to update,” shared the developer.