Even if the interstellar object known as Oumuamua were an interstellar lightsail, it could never travel fast enough to be practical, says a new paper.




The recent announcement that scientists have made human-monkey embryos and cultured them in the lab for two weeks made international headlines.
The technology to make animals that contain cells from other species has been available for decades and used extensively in research. These organisms are called “chimeras”.
But this latest advance highlights the need to broaden the discussion around the possible benefits of such research and, specifically, how inter-species chimeric research should be conducted in future.

It’s that time of year where the federal holiday in the United States commemorating the Declaration of Independence of the United States, (on July 4, 1776) is celebrated. To all my American fans I hope you have an awesome day! To celebrate I’m listing my favorite superheroes to rock the “Red, White and Blue”.
Happy Independence Day (Fourth of July)!
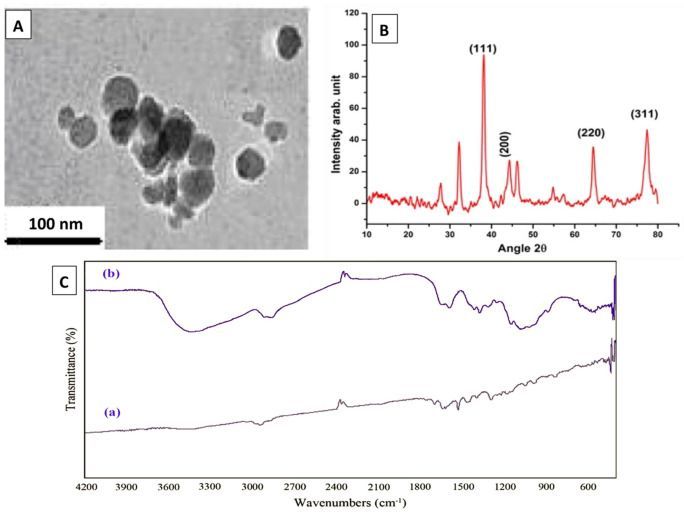
The adhesion and colonization or biofilm formation include primary stage in bacterial infections. Major adhesion virulence factors in this step include type I fimbriae (FimH) and pilli structures for attachment to the host cells7,8. Furthermore, numerous bacteria secrete toxins and extracellular enzymes which play a crucial role in the apoptosis or necrosis of epithelial cells or immunocytes. Various virulence factors of A. baumannii such as adhesins genes like kpsMII (group 2 capsule synthesis) and fimH, tratT (serum resistance associated), fyuA (yersiniabactin receptor) and iutA (aerobactin receptor) have been investigated previously9,10. An important polysaccharide for biofilm formation is encoded by pgaABCD locus11. Biofilm production is a strategy to escape from harsh conditions and immune responses, hence play as reservoirs for drug-resistant systemic infections. Biofilm-producing A. baumannii has been isolated from several infectious origins such as pneumonia and devise-associated infections. Bacterial within biofilm can resist significantly more against antibiotics compared to planktonic mode of growth12. Hence, biofilm-mediated infections are in relapse more frequently13.
Therefore, there is an urgent need to enhance the effects of antimicrobials against pathogenic bacteria. In recent years, interest has enhanced towards application of nanoparticles as therapeutic regimens14,15,16,17,18,19,20,21. Silver nanoparticles (AgNPs), which have low toxicity in ecosystems and have high rate of surface capacity, can inhibit accumulation of biofilm materials responsible for evasion and protection22,23,24.
The aim of this study was to isolate A. baumannii from wound infections, determine their resistance and virulence profile, and assess the impact of AgNPs on the bacterial growth, virulence and biofilm-related gene expressions in the isolated strains.

A new topic a new challenge for future civilizations.
I won’t write an introduction I will ask couple of questions to make you think about it.
In the forth industrial revolution are we going to change the way we reproduce? Could be the first step for post-human era in 2040?
How can we change the way we deal with economics? Because economy depends on population grow. In a way or another world population will stop at 11 billion so it is necessary to change the economy.
How can we colonize and expand while fertility rate is going down could another creatures like machines and biological engineered animals help us to expand and the human specie will limit its population?
Android malware known as FluBot is continuing to cause mayhem across some European countries, and there is speculation that the threat actors behind it may decide to target other geographies, including the United States. Here’s why you should be vigilant, how FluBot operates, and how you can remove this Android nasty from your device.
It’s also worth noting that this advice will help you stay safe from other Android malware strains. In recent days, cybercriminals have begun to target Europeans with TeaBot (also known as Anatsa or Toddler), an Android malware family that uses exactly the same technique as FluBot to spread and to lure users into giving up their sensitive data. FluBot and TeaBot are detected by ESET products as variants of the Android/TrojanDropper. Agent family.
I could probably be described as a SpaceX enthusiast. I catch their launches when I can, and I’ve watched the development of Starship with great interest. But the side-effect of SpaceX’s reusable launch system is that getting to space has become a lot cheaper. Having excess launch capacity means that space projects that were previously infeasible become suddenly at least plausible. One of those is Starlink.
Starlink is SpaceX’s satellite Internet service. Wireless and cellular internet have helped in some places, but if you really live out in the sticks, satellite internet is your only option. And while satellite Internet isn’t exactly new, Starlink is a bit different. Hughesnet, another provider, has a handful of satellites in geostationary orbit, which is about 22000 miles above the earth. To quote Grace Hopper, holding a nearly foot-long length of wire representing a nanosecond, “Between here and the satellite, there are a very large number nanoseconds.”
SpaceX opted to do something a bit different. In what seemed like an insane pipe dream at the time, they planned to launch a satellite constellation of 12000 birds, some of them flying as low as 214 mile altitude. The downside of flying so low is that they won’t stay in orbit as long, but SpaceX is launching them significantly faster than they’re coming down. So far, nearly 1600 Starlink satellites are in orbit, in a criss-crossing pattern at 342 miles (550 km) up.
How do simple creatures manage to move to a specific place? Artificial intelligence and a physical model from TU Wien can now explain this.
How is it possible to move in the desired direction without a brain or nervous system? Single-celled organisms apparently manage this feat without any problems: for example, they can swim towards food with the help of small flagellar tails.
How these extremely simply built creatures manage to do this was not entirely clear until now. However, a research team at TU Wien (Vienna) has now been able to simulate this process on the computer: They calculated the physical interaction between a very simple model organism and its environment. This environment is a liquid with a non-uniform chemical composition, it contains food sources that are unevenly distributed.