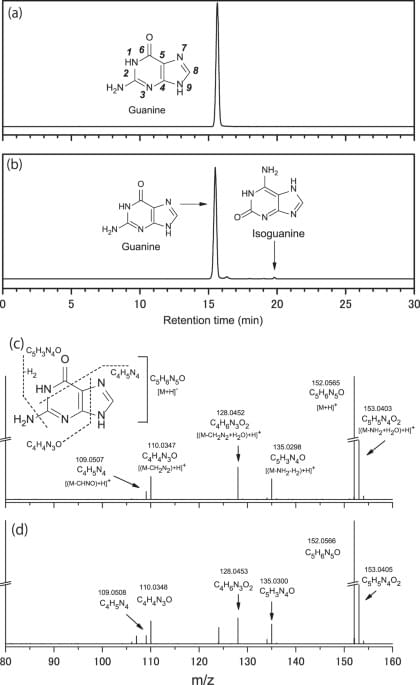
All DNA/RNA nucleobases were identified in carbonaceous meteorites. Having been provided to the early Earth as a component in carbonaceous meteorites, these molecules might have played a role for the emergence of genetic functions in early life.


Experimental observations conclude learning is mainly performed by neural … See more.
Summary: Experimental observations conclude learning is mainly performed by neural dendrite trees as opposed to modifying solely through the strength of the synapses, as previously believed.
Source: Bar-Ilan University
The brain is a complex network containing billions of neurons. Each of these neurons communicates simultaneously with thousands of others via their synapses (links), and collects incoming signals through several extremely long, branched “arms,” called dendritic trees.
For the last 70 years a core hypothesis of neuroscience has been that brain learning occurs by modifying the strength of the synapses, following the relative firing activity of their connecting neurons.
MIT researchers have developed a portable desalination unit, weighing less than 10 kilograms, that can remove particles and salts to generate drinking water.
The suitcase-sized device, which requires less power to operate than a cell phone charger, can also be driven by a small, portable solar panel, which can be purchased online for around $50. It automatically generates drinking water that exceeds World Health Organization quality standards. The technology is packaged into a user-friendly device that runs with the push of one button.
Unlike other portable desalination units that require water to pass through filters, this device utilizes electrical power to remove particles from drinking water. Eliminating the need for replacement filters greatly reduces the long-term maintenance requirements.


😀
A team of international researchers have identified a genetic cause of lupus. Researchers of the study pinpointed that DNA mutations in a gene that senses viral RNA represents one cause of the chronic condition, affecting approximately 1 in 1,000 people living in the UK. It is important to note that this genetic cause is not the sole trigger for everyone affected by lupus.
Researchers of the study sequenced the whole DNA genome of a juvenile systemic lupus erythematosus (JSLE) patient called Gabriela, who was diagnosed with severe lupus at the age of seven. A severe case such as this, with early onset of symptoms, is a rarity and is commonly associated with a single genetic cause, unlike adult-onset lupus.
The researchers that carried out the genetic analysis identified a single point mutation in the Toll Like Receptor 7 (TLR7) gene. Furthermore the researchers discovered other cases of severe lupus where this gene was also mutated.
One key aspect of intelligence is the ability to quickly learn how to perform a new task when given a brief instruction. For instance, a child may recognise real animals at the zoo after seeing a few pictures of the animals in a book, despite any differences between the two. But for a typical visual model to learn a new task, it must be trained on tens of thousands of examples specifically labelled for that task. If the goal is to count and identify animals in an image, as in “three zebras”, one would have to collect thousands of images and annotate each image with their quantity and species. This process is inefficient, expensive, and resource-intensive, requiring large amounts of annotated data and the need to train a new model each time it’s confronted with a new task. As part of DeepMind’s mission to solve intelligence, we’ve explored whether an alternative model could make this process easier and more efficient, given only limited task-specific information.
Today, in the preprint of our paper, we introduce Flamingo, a single visual language model (VLM) that sets a new state of the art in few-shot learning on a wide range of open-ended multimodal tasks. This means Flamingo can tackle a number of difficult problems with just a handful of task-specific examples (in a “few shots”), without any additional training required. Flamingo’s simple interface makes this possible, taking as input a prompt consisting of interleaved images, videos, and text and then output associated language.
Similar to the behaviour of large language models (LLMs), which can address a language task by processing examples of the task in their text prompt, Flamingo’s visual and text interface can steer the model towards solving a multimodal task. Given a few example pairs of visual inputs and expected text responses composed in Flamingo’s prompt, the model can be asked a question with a new image or video, and then generate an answer.

Earlier this month, we brought you the news that epigenetic reprogramming startup YouthBio Therapeutics had emerged from stealth. The company shed some light on its plans to develop epigenetic reprogramming therapies for age-related diseases by rejuvenating certain cells in our bodies. YouthBio aims to achieve this rejuvenation by developing gene therapies that enable partial cellular reprogramming – an area of longevity science that is now attracting significant commercial interest.
Longevity. Technology: Cellular reprogramming refers to the process of returning adult cells to a “pluripotent” state: blank, embryonic-like cells that can become any cell in the body. This reprogramming can be achieved using techniques based on the discovery of Yamanaka factors.
0:00 PeopleLens AI Helps The Blind.
1:40 Brain Fingerprints Detect Autism.
4:52 AI Predicts Cancer Tumor Regrowth.
Learn more about the future of decentralized AI here:
SingularityNET AGIX Website — https://singularitynet.io
Developer Documentation — https://dev.singularitynet.io/
Publish AI Services — https://publisher.singularitynet.io/
AGIX Community Telegram — https://t.me/singularitynet
AGIX Price Chat Telegram — https://t.me/AGIPriceTalk
SingularityDAO Dynamic Asset Sets: https://bit.ly/3wzr00o.
SingularityDAO AI DeFi Website — https://bit.ly/3npymhA
SingularityDAO AI DeFi App — https://bit.ly/3K9TvWM
SingularityDAO Twitter — https://twitter.com/SingularityDao.
SingularityDAO Medium — https://medium.com/singularitydao.
SingularityDAO Telegram — https://t.me/SingularityDAO
#AI #News #ML
Planetary Protection — how strict should the guidelines be to protect our home planet, to protect other worlds?
For more info see:
What’s First? A Life-Detection or a Human Mission to Mars?

Not even death itself will prevent this tech-savvy grandfather from meeting his future great-grandchildren.
Not even death will stop this tech-savvy grandfather from meeting his great-grandchildren.
Jerry Terrance, an 85-year-old grandfather from Los Angeles, California, has turned himself into a 3D hologram that will serve as a humanoid time capsule for future generations. According to the Daily Mail, Jerry’s ‘hologram twin’ will guide his two children and four grandchildren, as well as future great-grandchildren, through his family’s history, even after his death.
“I think it is a wonderful way to preserve my family’s history for future generations,” said Jerry while speaking to Jam Press. “To see myself like that, is just mind-blowing — it feels like watching a movie. By not just reading the words as in my memoir but to actually get the chance to see and hear me recalling the stories is just magical.”
Developed by StoryTerrace in partnership with 8i, the 3D hologram was captured in a custom green screen studio using 30 individual cameras. As Jerry thoughtfully recites his family history, accompanying home videos and photos are projected on the wall behind him. While explaining his creation of the Carpet Bag handbag in the 1960s, for example, we see some of the original promotional material for the item displayed throughout his virtual environment.