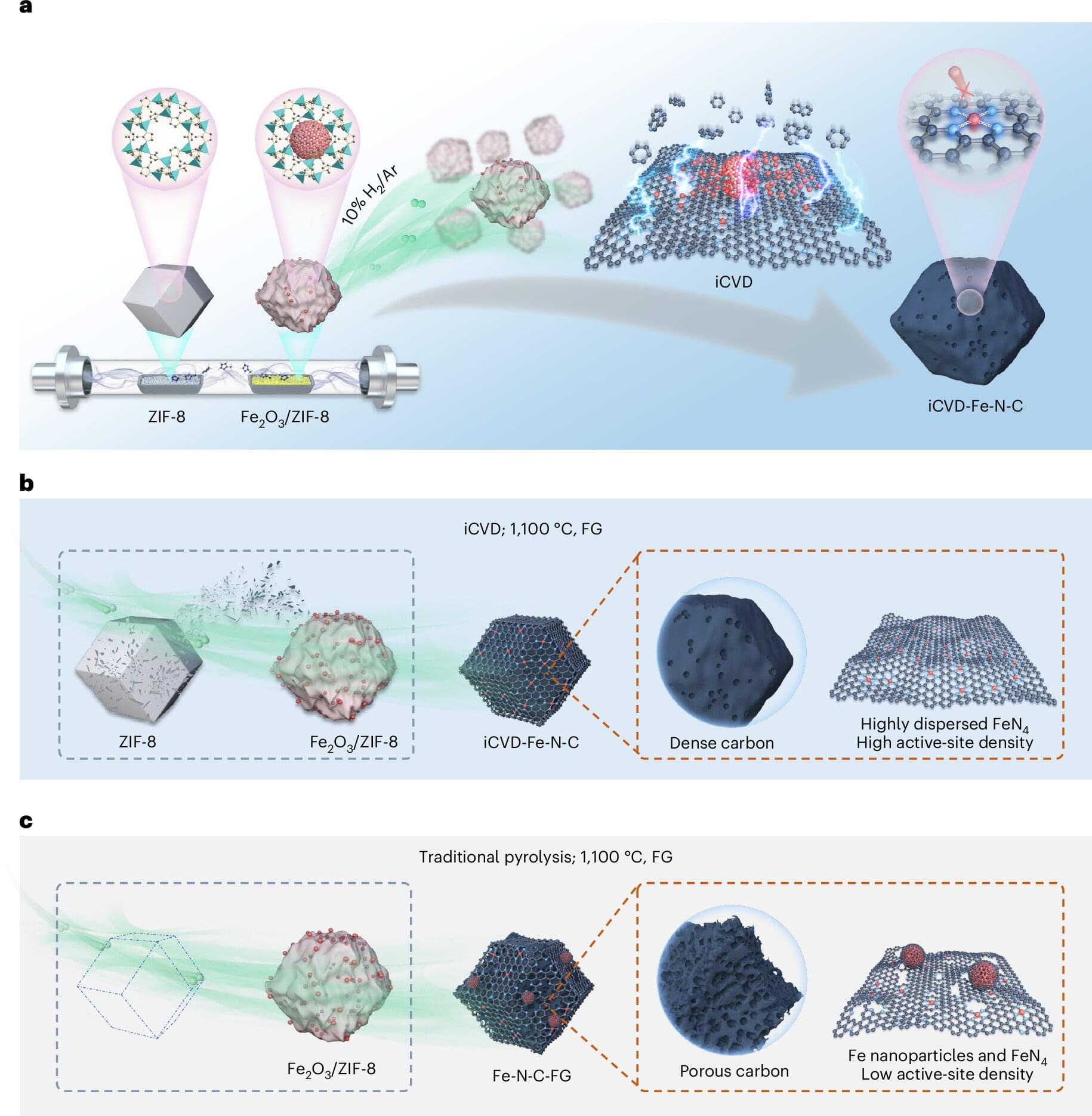
Japan and California have embraced hydrogen fuel-cell technologies, a form of renewable energy that can be used in vehicles and for supplying clean energy to manufacturing sectors. But the technology remains expensive due to its reliance on precious metals such as platinum. Engineers at Washington University in St. Louis are working on this challenge, finding ways to stabilize ubiquitous iron components for use in fuel cells to replace the expensive platinum metals, which would make hydrogen fuel-cell vehicles more affordable.
Cost challenges for fuel-cell vehicles
“The hydrogen fuel cell has been successfully commercialized in Japan and California in the U.S.,” said Gang Wu, a professor of energy, environmental and chemical engineering at the McKelvey School of Engineering. “But these vehicles struggle to compete with the battery vehicle and combustion engine vehicle, with cost being the main issue.”