A new study suggests that a child’s gut microbiome at age 2 may influence their emotional health years later.



How does the brain take out its trash? That is the job of the brain’s lymphatic drainage system, and efforts to understand how it works have pushed the boundaries of brain-imaging technologies.
A new study in iScience by researchers at the Medical University of South Carolina reveals—for the first time in humans—evidence of a previously unrecognized hub in the brain’s lymphatic drainage system—the middle meningeal artery (MMA).
Taking advantage of a NASA partnership that provided access to real-time MRI technologies originally developed to study how spaceflight affects fluid dynamics in the human brain, the MUSC research team, led by Onder Albayram, Ph.D., tracked cerebrospinal and interstitial fluid flow along the MMA in five healthy participants over a six-hour period. They found that the drainage flow of the cerebrospinal fluid was passive, suggesting lymphatic rather than blood flow. Blood would have had a faster, more dynamic flow.
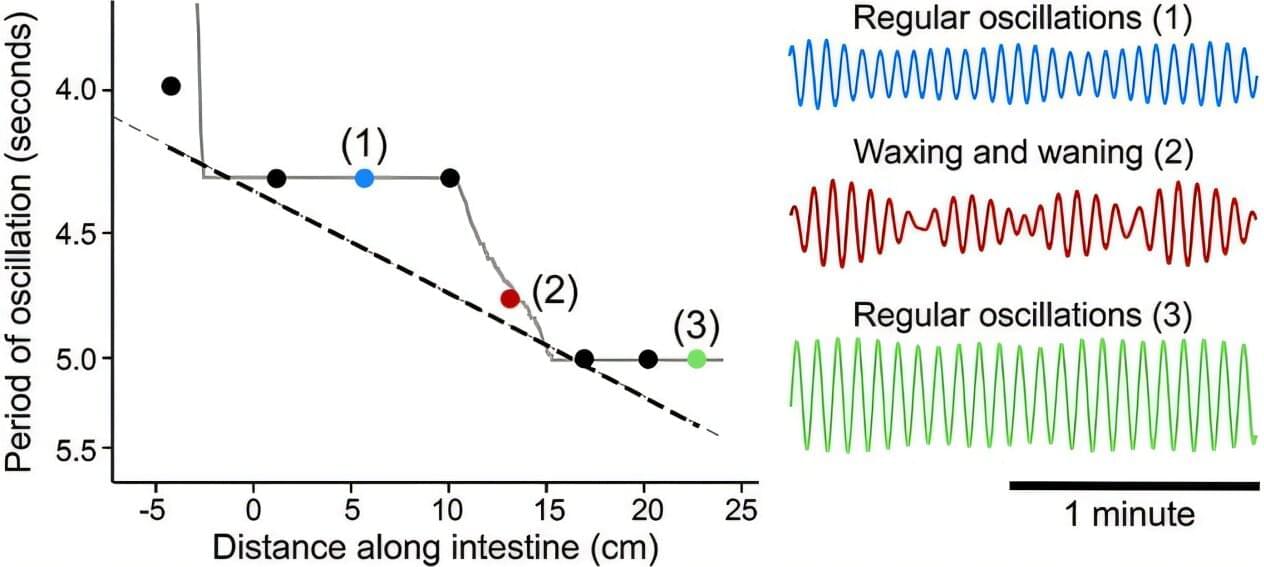
Synchronization abounds in nature: from the flashing lights of fireflies to the movement of fish wriggling through the ocean, biological systems are often in rhythmic movement with each other. The mechanics of how this synchronization happens are complex.
For instance, in the vasculature of the brain, blood vessels oscillate, expanding and contracting as needed. When there is neural activity, the arterioles expand to increase blood flow, oxygen and nutrients. These oscillations are self-sustained, but the arterioles also work in concert with each other. How this happens is not well understood.
To uncover the answer, researchers at the University of California San Diego looked to another part of the body: the gut. Here they found that oscillators operating at similar frequencies lock onto each other in succession, creating a staircase effect. Their work appears in Physical Review Letters.
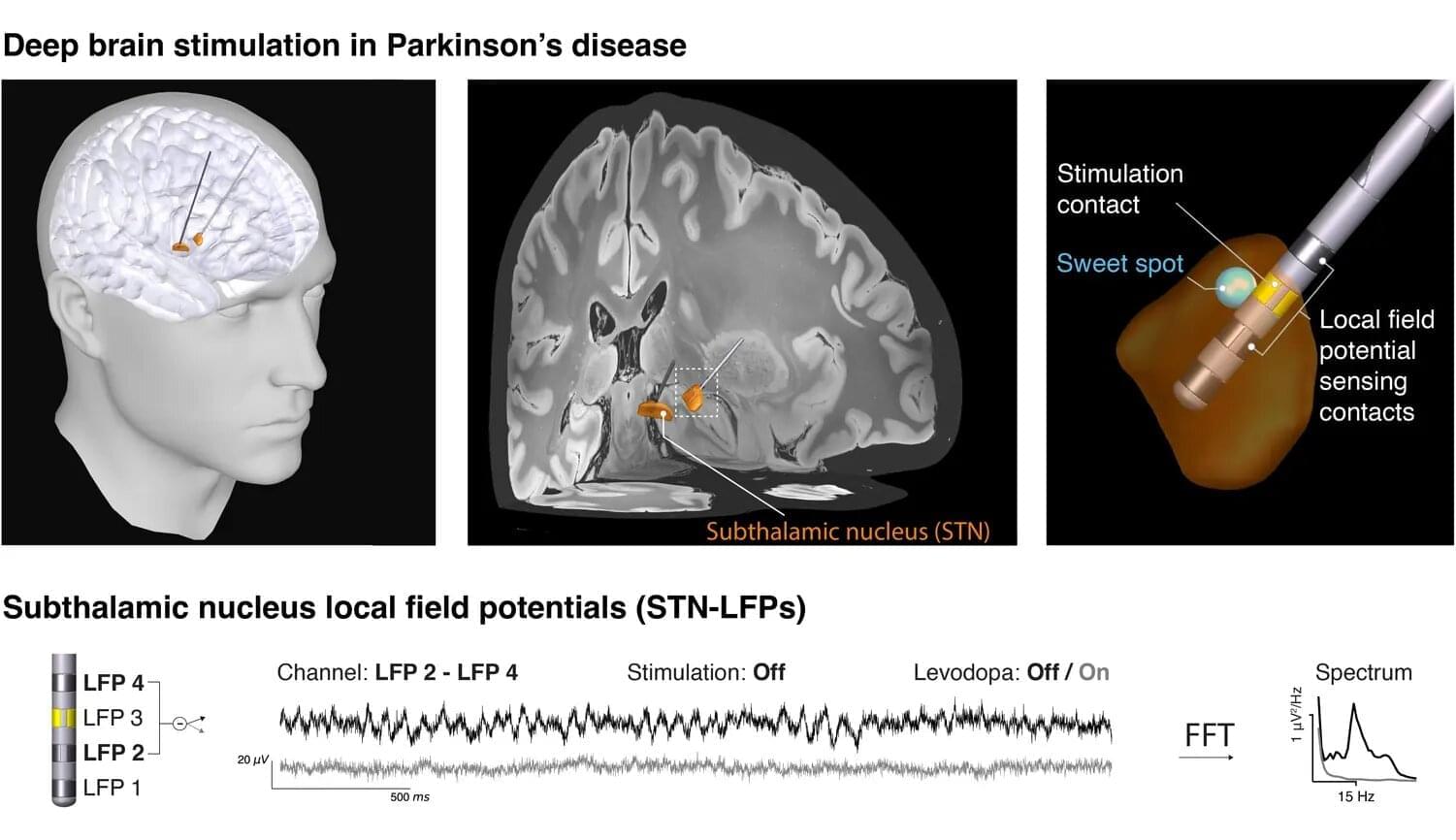
What happens in the brain when a person experiences the characteristic movement symptoms of Parkinson’s disease? Researchers around the world are seeking answers through various approaches. One of these builds on a treatment already established in clinical care: deep brain stimulation. In this therapy, stimulating electrodes are implanted in patients’ brains to alleviate symptoms using electrical impulses. The same electrodes also enable unique electrical measurements from areas otherwise inaccessible in humans. These data can help uncover the neural mechanisms of Parkinson’s disease and inspire new therapeutic strategies.
In close collaboration with leading European deep brain stimulation centers—including Charité Berlin, Heinrich-Heine University Düsseldorf, University College London, and the University of Oxford—the Max Planck team has now taken an important step forward. For their study, now published in eBioMedicine, the researchers focused on so-called “beta waves,” which oscillate ca. 20 times per second and whose strength is thought to correlate with the severity of movement symptoms.
However, when reviewing the literature, the team encountered considerable heterogeneity in the results. “We wondered why earlier studies from different centers had produced such mixed results,” says Vadim Nikulin of the Max Planck Institute for Human Cognitive and Brain Sciences in Leipzig. “Did the patient groups differ, the recording equipment, or the analysis methods?”

Chronic traumatic encephalopathy (CTE)—most often found in athletes playing contact sports—is known to share similarities with Alzheimer’s disease (AD), namely the buildup of a protein called tau in the brain.
New research published in Science finds even more commonalities between the two at the genetic level, showing CTE (like AD) is linked to damage to the genome and not just caused by repeated head impact (RHI).
The research team, a collaboration between Boston Children’s Hospital, Mass General Brigham, and Boston University, used single-cell genomic sequencing to identify somatic genetic mutations (changes in DNA that occur after conception and are not hereditary).
From mini-brains and spider-inspired gloves to edible wolf apple coatings and microplastic-filled retinas, scientists are transforming creepy concepts into life-improving innovations. Lab-grown brain organoids could replace animal testing, web-slinging gloves can spin instant wound dressings, and wolf apple starch may keep veggies fresh longer. Meanwhile, the discovery of microplastics in human eyes reveals a haunting truth about our environment’s reach inside us.
Lab-Grown “Mini-Brains” Offer New Insight into the Human Mind
Scientists writing in ACS Sensors have successfully grown a small brain organoid in a petri dish, creating a powerful new tool for studying how nerve cells interact without the use of animal testing. Over two years, human nerve cells multiplied and organized themselves into a three-dimensional “mini-brain” that displayed electrical activity similar to real brain tissue. Researchers say this breakthrough could help scientists better understand how the human brain communicates and functions—or, as they joke, provide “a lab-grown lunch option for zombies.”

Auditory-verbal hallucinations (AVH)—the experience of hearing voices in the absence of auditory stimulation—are a cardinal psychotic feature of schizophrenia-spectrum disorders. It has long been suggested that some AVH may reflect the misperception of inner speech as external voices due to a failure of corollary-discharge-related mechanisms. We aimed to test this hypothesis with an electrophysiological marker of inner speech.
Study Design.
Participants produced an inner syllable at a precisely specified time, when an audible syllable was concurrently presented. The inner syllable either matched or mismatched the content of the audible syllable. In the passive condition, participants did not produce an inner syllable. We compared the amplitude of the N1, P2, and P3-components of the auditory-evoked potential between: schizophrenia-spectrum patients with current AVH (SZAVH+, n = 55), schizophrenia-spectrum patients without current AVH (SZAVH−, n = 44), healthy controls (HC, n = 43).

The first distinction is between the notion of the level of consciousness and the notion of the contents of consciousness. In the first sense, consciousness is a property associated with an entire organism (a creature) or system: one is conscious (for example, when in a normal state of wakefulness) or not (for example, when in deep dreamless sleep or a coma). There is an ongoing vibrant debate about whether one should think of levels of consciousness as degrees of consciousness or whether they are best characterized in terms of an array of dimensions (11) or as “global states” (12). In the second sense, consciousness is always consciousness of something: our subjective experience is always “contentful”—it is always about something, a property philosophers call intentionality (3, 13). Here, again, there is some debate over the terms, for example, whether there can be fully contentless global states of consciousness (14) and whether consciousness levels (or global states) and contents are fully separable (11, 15).
The second distinction is between perceptual awareness and self-awareness (note that in this article, we use the terms consciousness and awareness interchangeably). Perceptual awareness simply refers to the fact that when we are perceptually aware, we have a qualitative experience of the external world and of our bodies within it (though of course, some perceptual experiences can be entirely fictive, such as when dreaming, vividly imagining, or hallucinating). Importantly, mere sensitivity to sensory information is not sufficient to be considered as perceptual awareness: the carnivorous plant Dionaea muscipula and the camera on your phone are both sensitive to their environment, but we have little reason to think that either has perceptual experiences. Thus, mere sensitivity is not sufficient for perceptual awareness, as it does not necessarily feel like something to be sensitive. This experiential character is precisely what makes the corresponding sensation a conscious sensation (16).
Researchers used supramolecular nanoparticles to repair the brain’s vascular system and reverse Alzheimer’s in mice. Instead of carrying drugs, the nanoparticles themselves triggered natural clearance of amyloid-β proteins. This restored blood-brain barrier function and reversed memory loss. The results point to a revolutionary new path for treating neurodegenerative diseases.
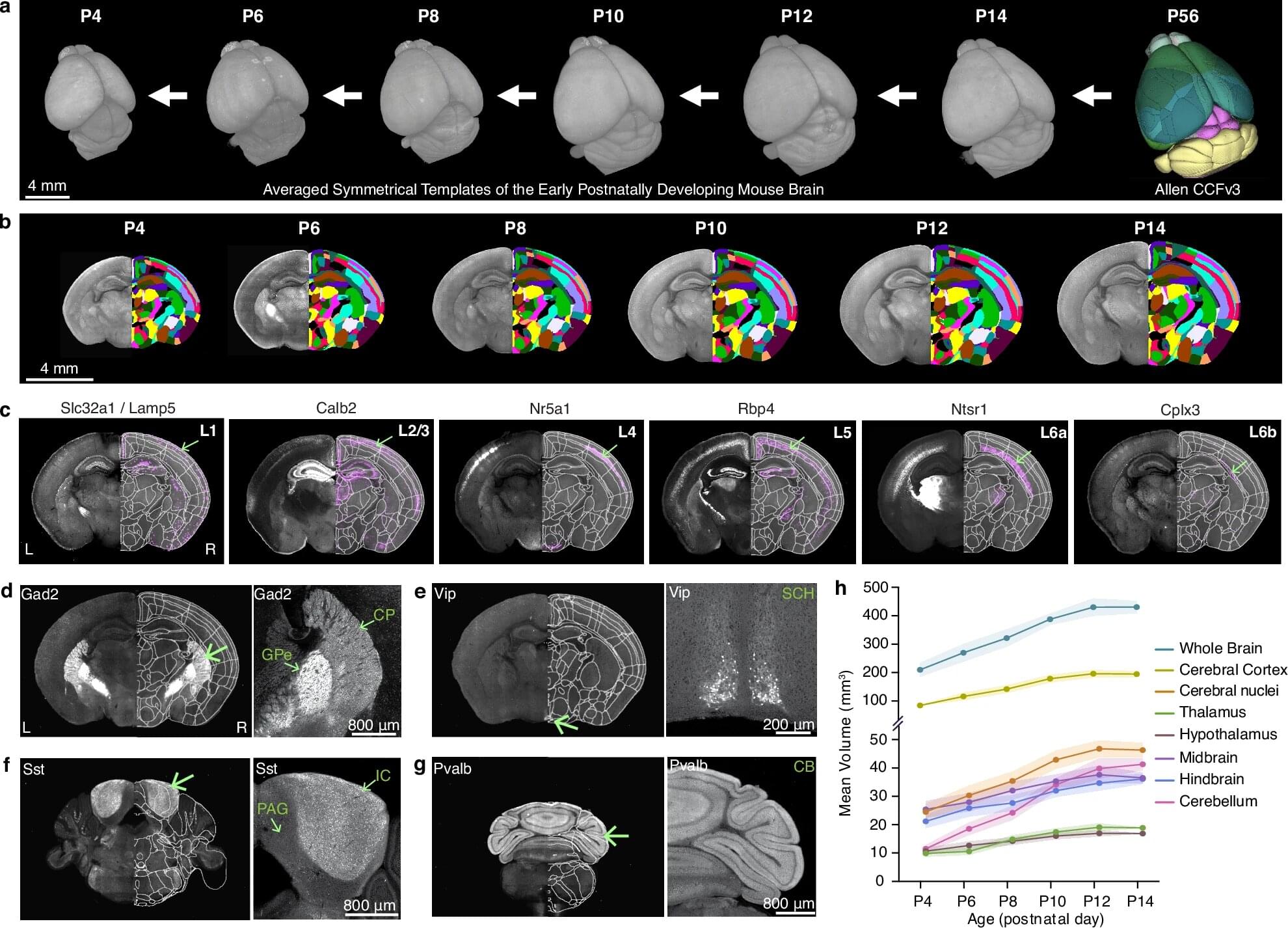
Brain growth and maturation doesn’t progress in a linear, stepwise fashion. Instead, it’s a dynamic, choreographed sequence that shifts in response to genetics and external stimuli like sight and sound. This is the first high-resolution growth chart to explain changes of key brain cell types in the developing mouse brain, led by a team at Penn State College of Medicine and the Allen Institute for Brain Science.
Using advanced imaging techniques, the researchers constructed a series of 3D atlases that are like time-lapsed maps of the brain during its first two weeks after birth, offering an unparalleled look at a critical period of brain development. It’s a powerful tool to understand healthy brain development and neurodevelopmental disorders, the researchers explained.
The study, published in Nature Communications, also detailed how regions of the brain change in volume and explained the shift in density of key cell types within them.