Psilocybin’s health benefits could extend beyond the mental realm.



Scientists at UCSF have uncovered how certain immune cells in the brain, called microglia, can effectively digest toxic amyloid beta plaques that cause Alzheimer’s. They identified a key receptor, ADGRG1, that enables this protective action. When microglia lack this receptor, plaque builds up quickly, causing memory loss and brain damage. But when the receptor is present, it seems to help keep Alzheimer's symptoms mild. Since ADGRG1 belongs to a drug-friendly family of receptors, this opens the door to future therapies that could enhance brain immunity and protect against Alzheimer’s in more people.

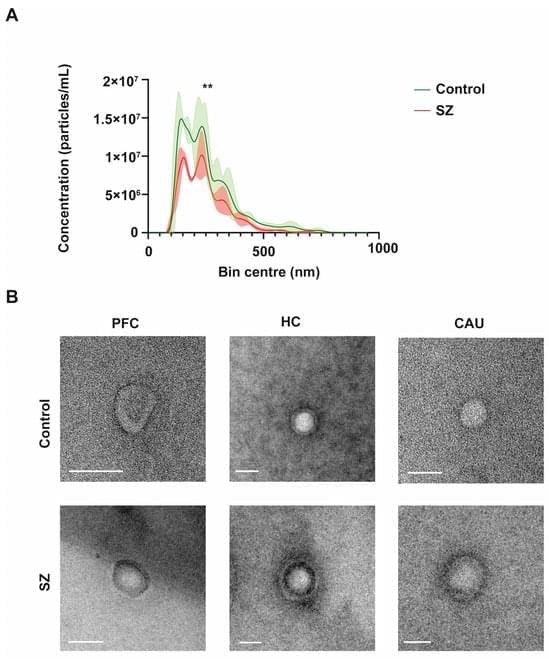
Extracellular vesicles (EVs) are tiny membranous structures that mediate intercellular communication. The role(s) of these vesicles have been widely investigated in the context of neurological diseases; however, their potential implications in the neuropathology subjacent to human psychiatric disorders remain mostly unknown. Here, by using next-generation discovery-driven proteomics, we investigate the potential role(s) of brain EVs (bEVs) in schizophrenia (SZ) by analyzing these vesicles from the three post-mortem anatomical brain regions: the prefrontal cortex (PFC), hippocampus (HC), and caudate (CAU). The results obtained indicate that bEVs from SZ-affected brains contain region-specific proteins that are associated with abnormal GABAergic and glutamatergic transmission.
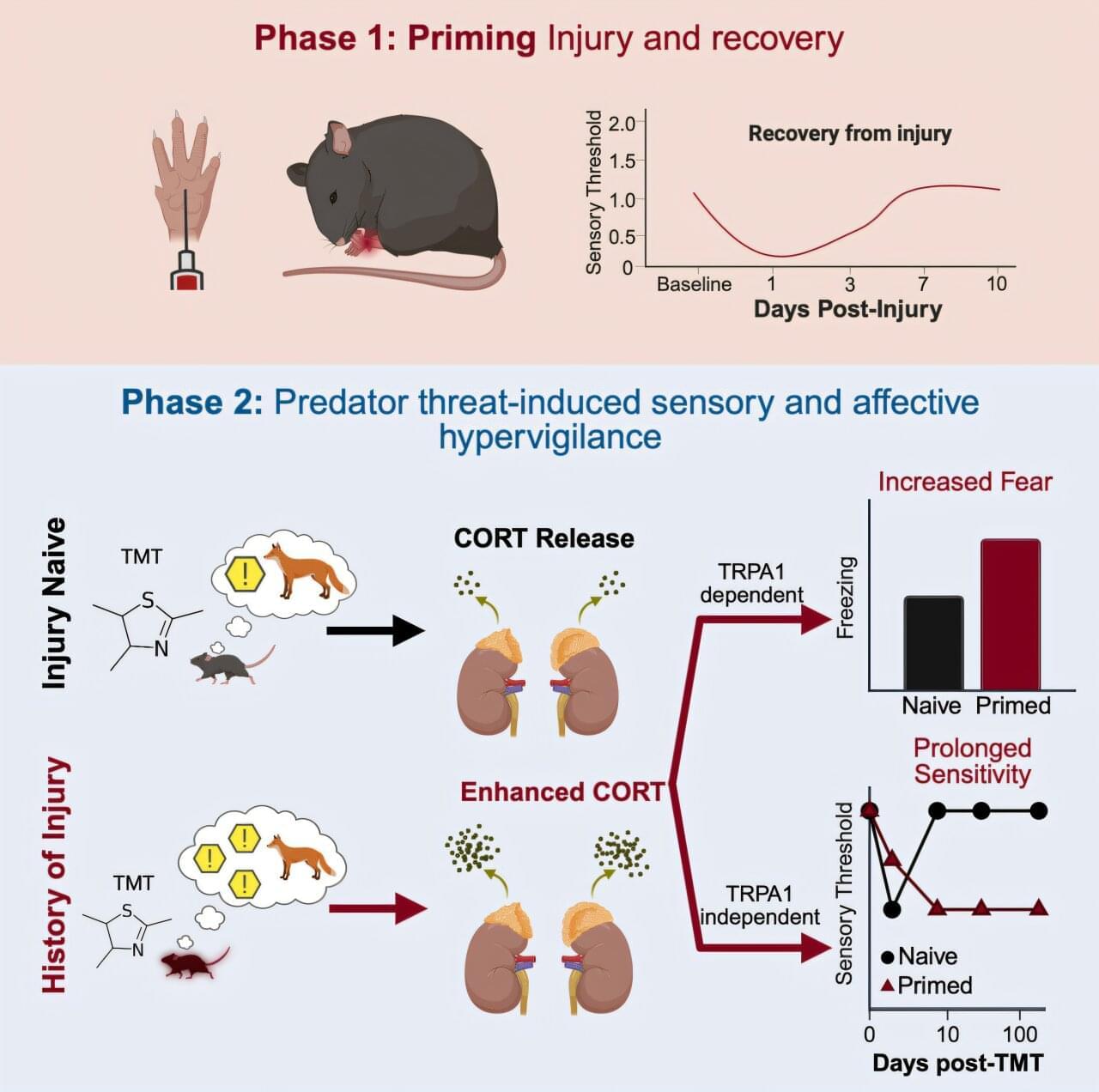
A wound can leave a lasting imprint—even after it has healed. A new study in Current Biology finds that past injuries can quietly prime the body to overreact and be more sensitive to stress, pain and fear long after the damage is gone.
These findings may help explain how early injuries or trauma can set the stage for chronic pain conditions, where the nervous system remains hypersensitive long after the initial damage has healed. can set the stage for chronic pain conditions, where the nervous system remains hypersensitive long after the initial damage has healed.
Researchers at the University of Toronto Mississauga discovered that mice with a history of injury responded more intensely to the scent of a predator, an extremely stressful event for mice. These mice showed exaggerated fear and developed long-lasting pain in both hind paws, including the uninjured side. Strikingly, the symptoms lasted more than six months, long after the original injury had physically healed.

Copper is an essential trace element for normal development and function throughout the body, including the central nervous system (CNS). Alterations to cellular copper levels result in severe neurological consequences and are linked to a range of CNS disorders, positioning treatments that restore copper balance as promising therapies for these disorders. However, despite the clear relationship between copper balance and CNS health, there are limited tools to measure copper levels in vivo in humans. This constitutes a significant challenge for both diagnosing disorders of copper imbalance and monitoring the efficacy of copper-altering treatments for these disorders. Here we report the synthesis and characterization of Fluorine-labeled Naphthalimide Copper sensor 1 (F-NpCu1), a fluorescent sensor for copper that contains a fluorine atom for future radiolabeling for clinical application. We demonstrate that the probe exhibits good stability and is highly selective for copper above other transition metals present in biological tissues. Copper binding promotes covalent bond formation between the sensor and proximal cellular proteins. F-NpCu1 is nontoxic and can be measured using fluorescence microscopy in living cells and fixed tissue sections from both mouse brain and pancreas. Furthermore, F-NpCu1 exhibits good blood-brain-barrier permeability and can report differences in brain copper levels induced by copper modulating therapies in living mice using intravital fluorescence microscopy. This study represents a promising advance toward the development of the first clinical tool for measuring copper in living humans, including in the CNS, with radiolabeling studies underway to develop 18F-NpCu1 for PET imaging of copper in vivo.
