Bio threat from China & PLA: Reports.
‘Neuro strike weapons being developed, population to be brainwashed; Tech weapon to disrupt brain functions,’ says report.
#china #xijinping #bioweapons #englishnews n18oc_breaking-news.
Bio threat from China & PLA: Reports.
‘Neuro strike weapons being developed, population to be brainwashed; Tech weapon to disrupt brain functions,’ says report.
#china #xijinping #bioweapons #englishnews n18oc_breaking-news.
According to intelligence analysts, China’s People’s Liberation Army is reportedly developing high-technology neurostrike weapons that are designed to disrupt brain functions and influence government leaders or entire population. The weapons can be used to directly attack or control brains using microwave or other directed energy weapons in handheld guns or larger weapons firing electromagnetic beams. Analysts, in their report, say that the danger of China’s brain warfare weapons prior to or during a conflict is no longer theoretical. They are also of the opinion that China‘s leadership views neurostrike and psychological warfare as a core component of its asymmetric warfare strategy against the United States and its allies in the Indo-Pacific. Neurostrike is a military term defined as the engineered targeting of the brains of military personnel or civilians using non-kinetic technology. The goal is to impair thinking, reduce situational awareness, inflict long-term neurological damage and cloud normal cognitive functions.
#Neurostrikeweapons #Chinaneurostrike #Chinaneweapon.
~PR.153~ED.102~HT.96~
Oneindia News is a youth-driven channel that brings you stories that need your attention, are popular, informative, and entertaining. Follow and like us for thought-provoking & exclusive content…
Youtube:
Like us on Facebook: https://www.facebook.com/oneindianews.
Follow us on Instagram: https://www.instagram.com/oneindia_news.
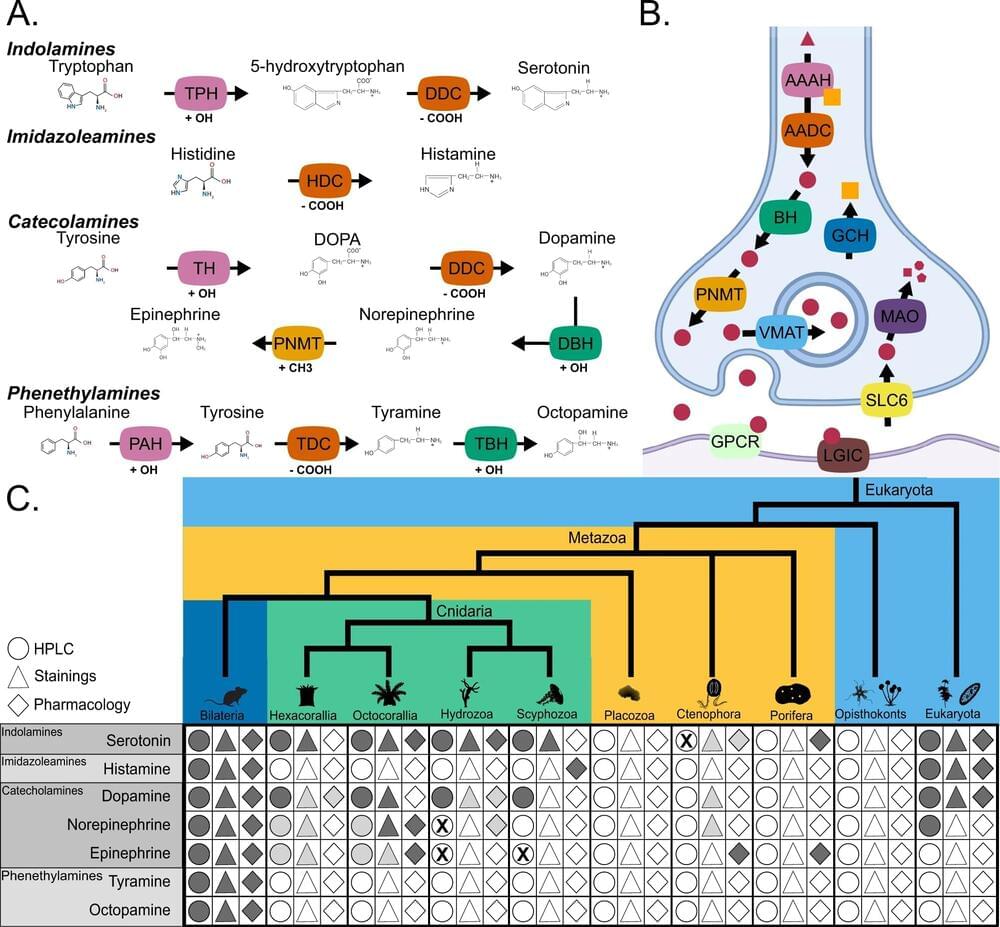
A team of scientists led by researchers from the University of Leicester have discovered that the genes required for learning, memory, aggression and other complex behaviors originated around 650 million years ago.
The findings led by Dr. Roberto Feuda, from the Neurogenetic group in the Department of Genetics and Genome Biology and other colleagues from the University of Leicester and the University of Fribourg (Switzerland), have now been published in Nature Communications.
Dr. Feuda said, “We’ve known for a long time that monoamines like serotonin, dopamine and adrenaline act as neuromodulators in the nervous system, playing a role in complex behavior and functions like learning and memory, as well as processes such as sleep and feeding.”
The Fine-Tuning Argument is often seen as the best argument for the existence of God. Here we have assembled some of the world’s top physicists and philosophers to offer a reply. Not every critic of the argument comes from the same perspective. Some doubt there is a problem to be solved whilst others agree it is a genuine problem but think there are better solutions than the God hypothesis. Some like the multiverse and anthropics other don’t. We have tried to represent these different approaches and so it should be taken as given, that not all of the talking heads agree with each other. Nevertheless, they all share the view that the fine-tuning argument for God does not work. Nor are all the objectors atheist, Hans Halvorson offers what we think is a strong theological objection to the argument. This film does not try to argue that God doesn’t exist only that the fine-tuning argument is not a good reason to believe in God. Most of the footage was filmed exclusively for this film with some clips being re-used from our Before the Big Bang series, which can be viewed here: https://www.youtube.com/watch?v=Ry_pILPr7B8&list=PLJ4zAUPI-q…4hnojoCR4m All of the critics of the fine tuning argument that appear were sent a draft of the film more than a month before release and asked for any objections either to their appearance, the narration or any other aspect of the film. No objections were raised, and many replies were extremely positive and encouraging. A timeline of the subjects covered is below:
(We define God as a perfect Omni immaterial mind as for example modern Christians and Muslims advocate, there are other conceptions of God which our video does not address).
Just to be clear, this is a polemical film arguing against the fine tuning argument.
Timecodes.
0:00 Introduction.
4:11 The universe as a roll of the dice.
6:15 what is probability?
7:28 probability problems.
9:25 measure problem.
15:45 deceptive probabilities.
20:23 the flatness problem.
22:14 counterfactuals versus probabilities.
23:59 fine tuning versus God.
37:02 necessity.
38:53 multiverse and anthropics.
47:34 Boltzmann brains.
49:45 Entropy.
52:45 Cosmological Natural Selection.
59:10 conclusion.

Breast milk is not simply sustenance. It also is rich in micronutrients that are critical for healthy brain development in infants.
Now, researchers have identified a component of breast milk that promotes how neurons form connections in infants’ brains. Myo-inositol is a small cyclic sugar molecule in breast milk that also is found in a typical adult diet, including in fruits and grains. The study emphasizes the powerful role that what we eat plays in brain function. It was published in PNAS on July 11.
“The effects of micronutrients on the brain are really under-appreciated,” says Thomas Biederer, Ph.D., associate professor of neurology and principal investigator. “As a neuroscientist, our findings were stunning to me.”

Summary: A new study reveals that individuals with stronger ‘mindreading’ abilities, or the capacity to understand others’ feelings and intentions, are more successful in cooperative tasks. This trait, also known as ‘theory of mind,’ is not directly tied to intelligence and can potentially be improved through training programs.
The research demonstrated that those with high theory of mind were more effective in cooperation, particularly when paired with individuals with similar abilities. The study underlines the potential to foster enhanced cooperation in various settings like schools, workplaces, or colleges by improving these abilities.
Prosthetics moved by thoughts. Targeted treatments for aggressive brain cancer. Soldiers with enhanced vision or bionic ears.
These powerful technologies sound like science fiction, but they’re becoming possible thanks to nanoparticles.
And, as with any great power, there comes great responsibility.

Summary: Researchers have uncovered genes essential for learning, memory, aggression, and other complex behaviors originated around 650 million years ago.
The study utilized computational methods to trace the evolutionary history of these genes involved in the production, modulation, and reception of monoamines like serotonin, dopamine, and adrenaline. This discovery suggests that this new method of modulating neuronal circuits could have played a role in the Cambrian Explosion, contributing to the diversification of life.
The finding offers new research avenues to understand the origins of complex behaviors and their relation to diverse processes like reward, addiction, aggression, feeding, and sleep.
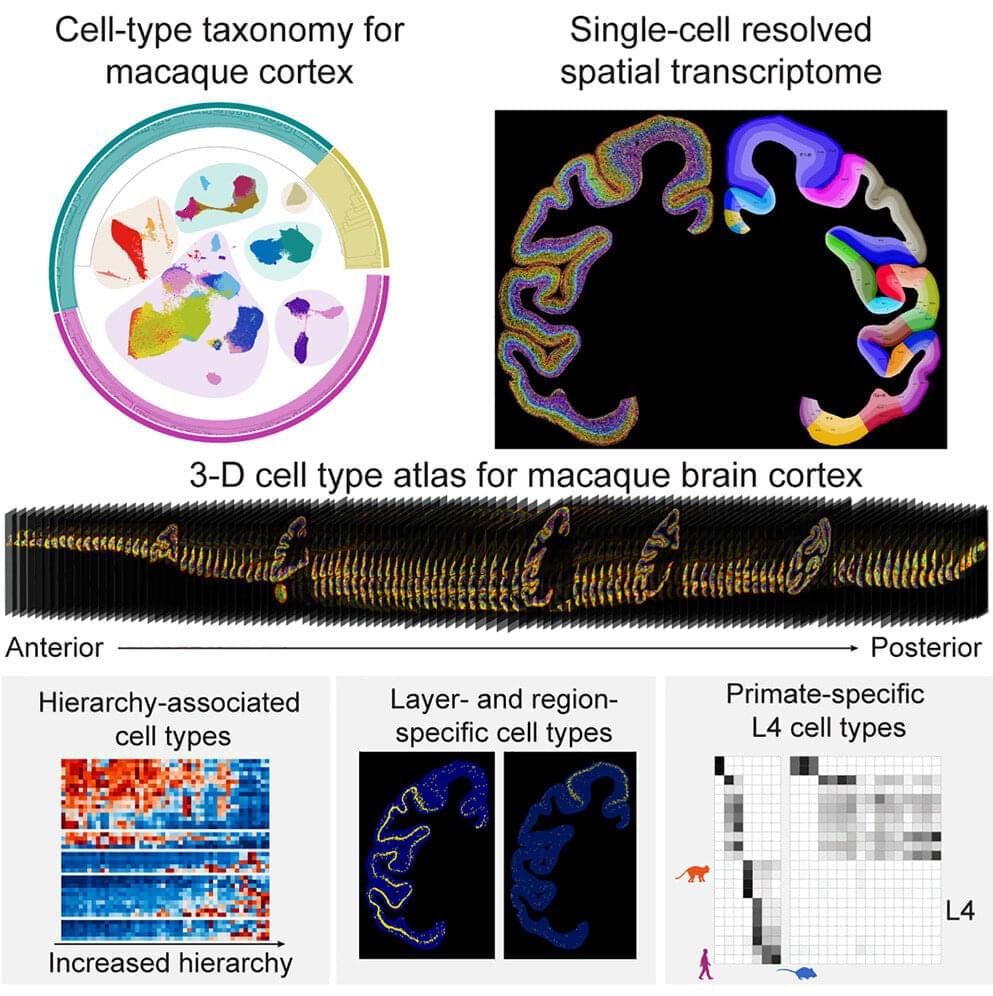
A team of nearly 100 scientists recently mapped the cell-type taxonomy in the macaque cortex and revealed the relationship between cell-type composition and various primate brain regions by using the self-developed spatial transcriptome sequencing technology Stereo-seq and snRNA-seq technology, which provides a molecular and cellular basis for further investigation into neural circuits.
The study was published in Cell.
Primates have a vast number of neurons that form complex and intricate neural circuits supporting advanced cognition and behavior. Disruptions in these cells and circuits can lead to various brain disorders. Understanding the composition and spatial distribution of cells in the brain, as well as the relationships between them, is a fundamental question in neuroscience, comparable to the periodic table in chemistry, the world map in geographic discoveries, or the DNA base sequence discovered through human genome sequencing.