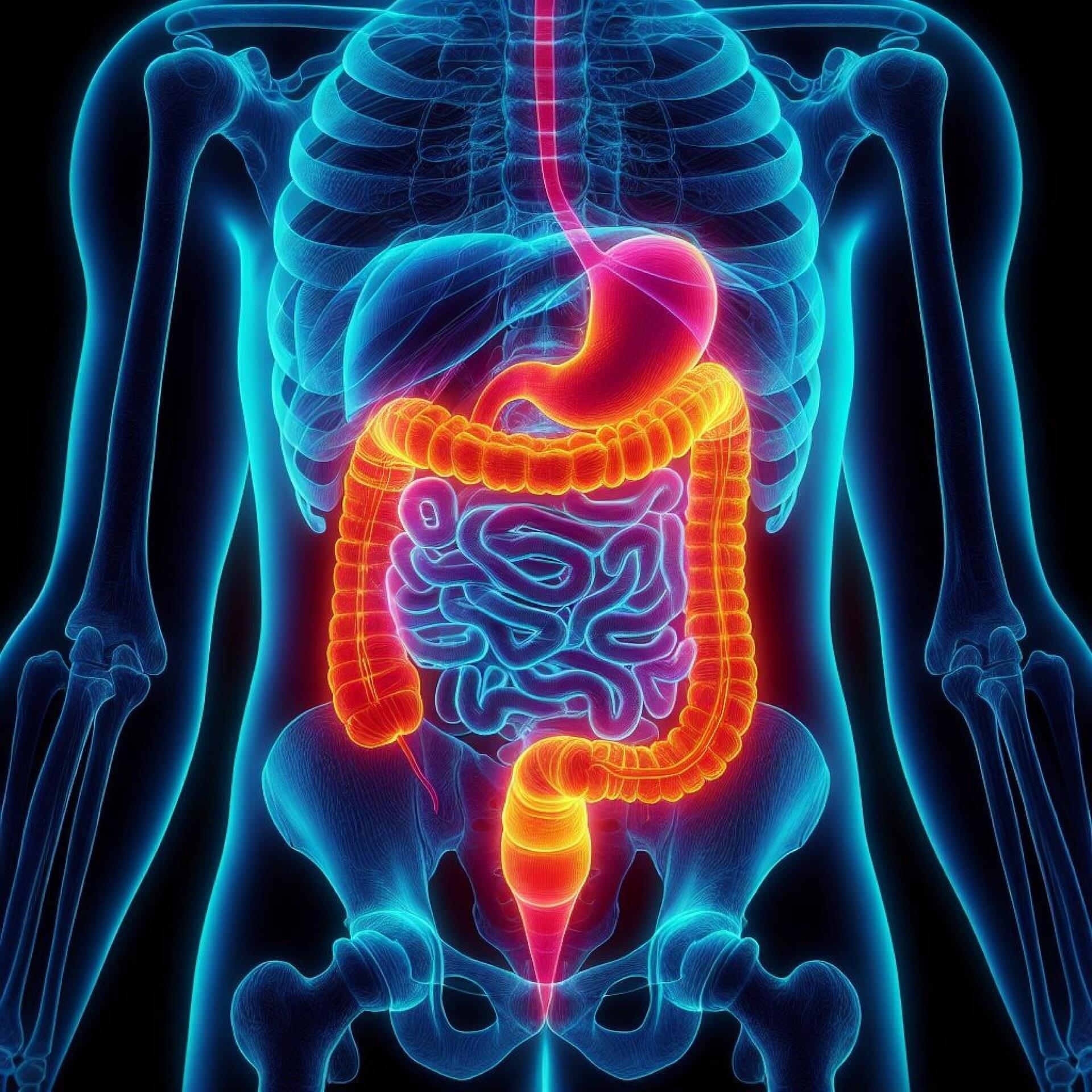
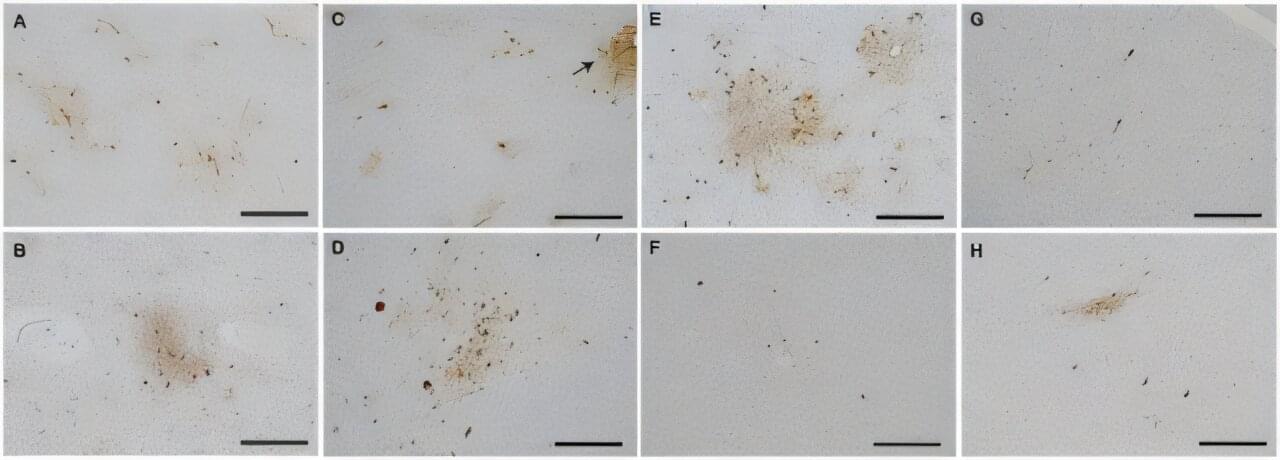
Australian researchers have identified two nervous system components that drive tumor growth in gastrointestinal cancers, creating promising new avenues for treatment with existing approved therapies.
Our gut contains its very own nervous system and is commonly regarded as the second brain. Key players of this system are neuropeptides, the signaling factors that are produced and released by nerves. These factors relay messages throughout our nervous system by connecting to receptors on the outside of cells, influencing a variety of processes.
The team at the Olivia Newton-John Cancer Research Institute (ONJCRI) and La Trobe School of Cancer Medicine discovered that CGRP, a common neuropeptide, and its receptor RAMP1 influence tumor growth in colorectal and stomach cancers.