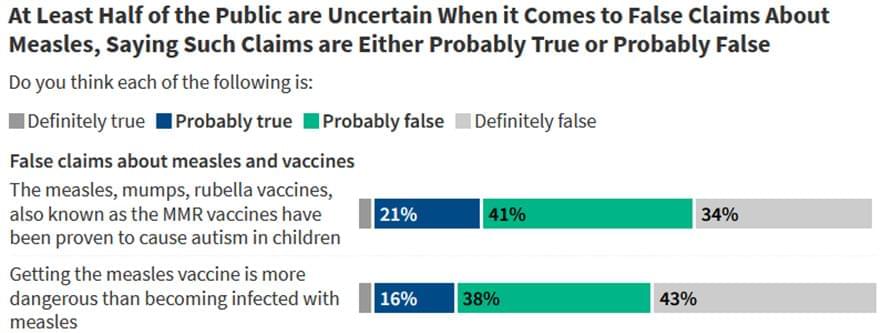
Researchers at Karolinska Institutet in Sweden have identified a brain circuit that can drive repetitive and compulsive behaviors in mice, even when natural rewards such as food or social contact are available. The study has been published in the journal Science Advances and may contribute to increased knowledge about obsessive-compulsive disorder and addiction.
Both animals and humans can become stuck in certain behaviors, but exactly how this is regulated in the brain has been unknown. Now, researchers have been able to show that a specific nerve circuit in the brain can put behaviors into a kind of “repeat mode,” where mice continue to perform the same actions over and over again, even when there is no longer any reward.
The researchers investigated a neural circuit that runs from the nucleus accumbens, part of the brain’s reward system, to a region in the hypothalamus, which in turn is connected to the lateral habenula, an area that processes unpleasant experiences. By activating this circuit using optogenetics, a method in which nerve cells are controlled by light, the researchers were able to induce a negative state in mice that led to repetitive behaviors such as digging and sniffing—even when food or other rewards were available.