The MIND diet, focusing on brain-healthy foods like leafy greens and berries, is linked to a lower risk of Alzheimer’s and dementia, according to recent studies.



More and more people are investing their time and energy into longevity — it’s not just living longer, but living happier, healthier and staying productive well past what has been considered “old age.” McKinsey reports that up to 60 percent of consumers across health and wellness markets say that healthy aging is a “top” or “very important” priority. The movement has created a boost in the health and wellness businesses, and to get an overview of the longevity supplements space, we spoke with Dr. Luke Winegard, the Chief Operating Officer at Longevity Method.
Entrepreneur: What is driving the current boom in the longevity supplement market? Dr. Luke Winegard: Growing consumer demand for health and wellness products is creating explosive growth in the longevity supplement market. Scientific advancements and increasing health consciousness are driving this trend, with consumers now focused on “healthspan” — not just how long they live, but how well they live. The pursuit of longevity has moved from being a niche interest of visionaries to becoming mainstream in 2025.
What does “healthspan” mean and why is it important? Healthspan refers to the period of life spent in good health, free from chronic diseases and disabilities. Today’s consumers are concerned not just about adding years to their lives, but making those years healthy, productive, and vital. This represents a cultural shift toward proactive self-optimization where maintaining energy, cognitive sharpness, and resilience is just as important as achieving physical goals.

The brainwaves of people with neuropathic pain show a distinct pattern: more slow theta waves, fewer alpha waves, and more fast, high beta waves, co-lead author Sylvia Gustin, a clinical psychologist and UNSW professor, said in the statement. Her research has investigated changes in the thalamus—a central brain structure that relays sensory and motor signals to the cerebral cortex—associated with nerve pain.
The PainWaive system consists of an electroencephalogram (EEG) headset that records brain activity paired with an app that instructs patients on how to control their brainwaves through neurofeedback games, according to a UNSW statement. Four participants who suffer from corneal neuropathic pain—a condition that causes painful hypersensitivity of the eyes, face, or head—underwent 20 PainWaive sessions over the course of four weeks.
This study offers new hope for drug-free pain treatments, but further trials will need to verify its results.

Nuri Jeong remembers the feeling of surprise she felt during a trip back to South Korea, while visiting her grandmother, who’d been grappling with Alzheimer’s disease.
“I hadn’t seen her in six years, but she recognized me,” said Jeong, a former graduate researcher in the lab of Annabelle Singer in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University.
“I didn’t expect that. Even though my grandmother struggled to remember other family members that she saw all the time, she somehow remembered me,” Jeong added. “It made me wonder how the brain distinguishes between familiar and new experiences.”

People who follow a MIND diet, even if started later in life, were significantly less likely to develop Alzheimer’s disease or related forms of dementia, according to new research.
The MIND diet stands for “Mediterranean-DASH Intervention for Neurodegenerative Delay” and combines many elements of the Mediterranean diet and DASH (“Dietary Approaches to Stop Hypertension”). It emphasizes brain-healthy foods like leafy greens, berries, nuts and olive oil.
The study, being presented Monday at the American Society for Nutrition’s annual meeting, analyzed data from nearly 93,000 U.S. adults aged 45 to 75 starting in the 1990s.
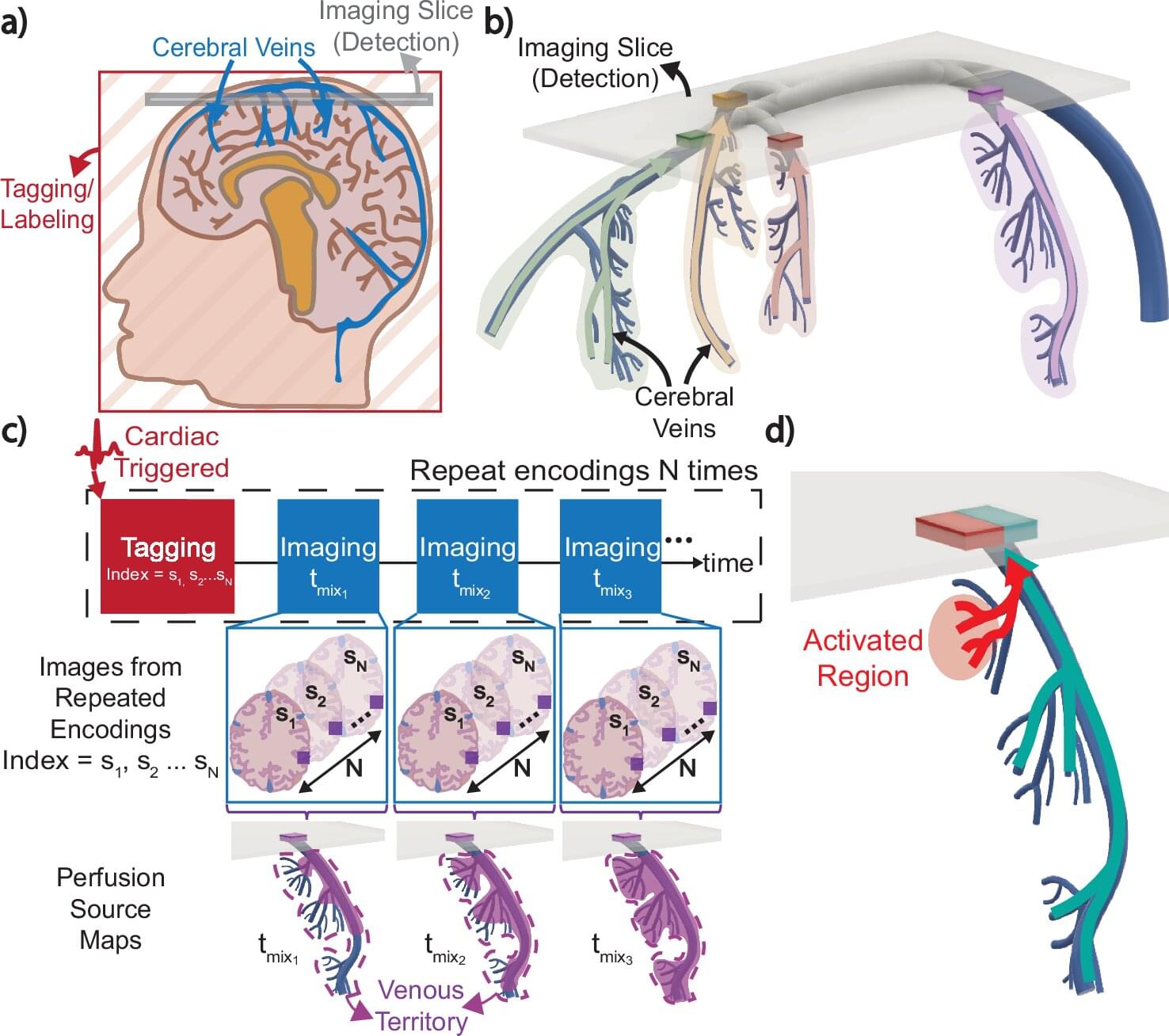
The venous system maintains the health of our brains by removing deoxygenated blood and other waste products, but its complexity and variability have made scientific study difficult. Now, a UC Berkeley-led team of researchers has developed an innovative MRI technique that may expand our understanding of this critical system.
In a study published in Nature Communications, the researchers demonstrate how their new imaging method, Displacement Spectrum (DiSpect) MRI, maps blood flows “in reverse” to reveal the source of blood in the brain’s veins. This approach could help answer long-standing questions about brain physiology as well as provide a safer, more efficient way to diagnose disease.
Like some current MRI methods, DiSpect uses the water in our blood as a tracing agent to map perfusion, or blood flow, in the brain. The water’s hydrogen atoms possess a quantum mechanical property called spin and can be magnetized when exposed to a magnetic field, like an MRI scanner. But what makes DiSpect unique is its ability to track the “memory” of these nuclear spins, allowing it to map blood flow back to its source.

A trial of an interactive game that trains people to alter their brain waves has shown promise as a treatment for nerve pain—offering hope for a new generation of drug-free treatments.
The PainWaive technology, developed by UNSW Sydney researchers, teaches users how to regulate abnormal brain activity linked to chronic nerve pain, offering a potential in-home, noninvasive alternative to opioids.
A recent trial of the technology, led by Professor Sylvia Gustin and Dr. Negin Hesam-Shariati from UNSW Sydney’s NeuroRecovery Research Hub, has delivered promising results, published in the Journal of Pain.
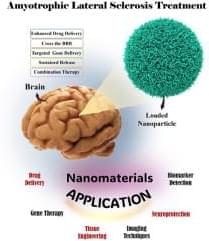
This review explores the transformative potential of nanotechnology in the treatment and diagnosis of amyotrophic lateral sclerosis (ALS), a progressive neurodegenerative disorder characterized by motor neuron degeneration, muscle weakness, and eventual paralysis. Nanotechnology offers innovative solutions across various domains, including targeted drug delivery, neuroprotection, gene therapy and editing, biomarker detection, advanced imaging techniques, and tissue engineering. By enhancing the precision and efficacy of therapeutic interventions, nanotechnology facilitates key advancements such as crossing the blood-brain barrier, targeting specific cell types, achieving sustained therapeutic release, and enabling combination therapies tailored to the complex pathophysiology of ALS.
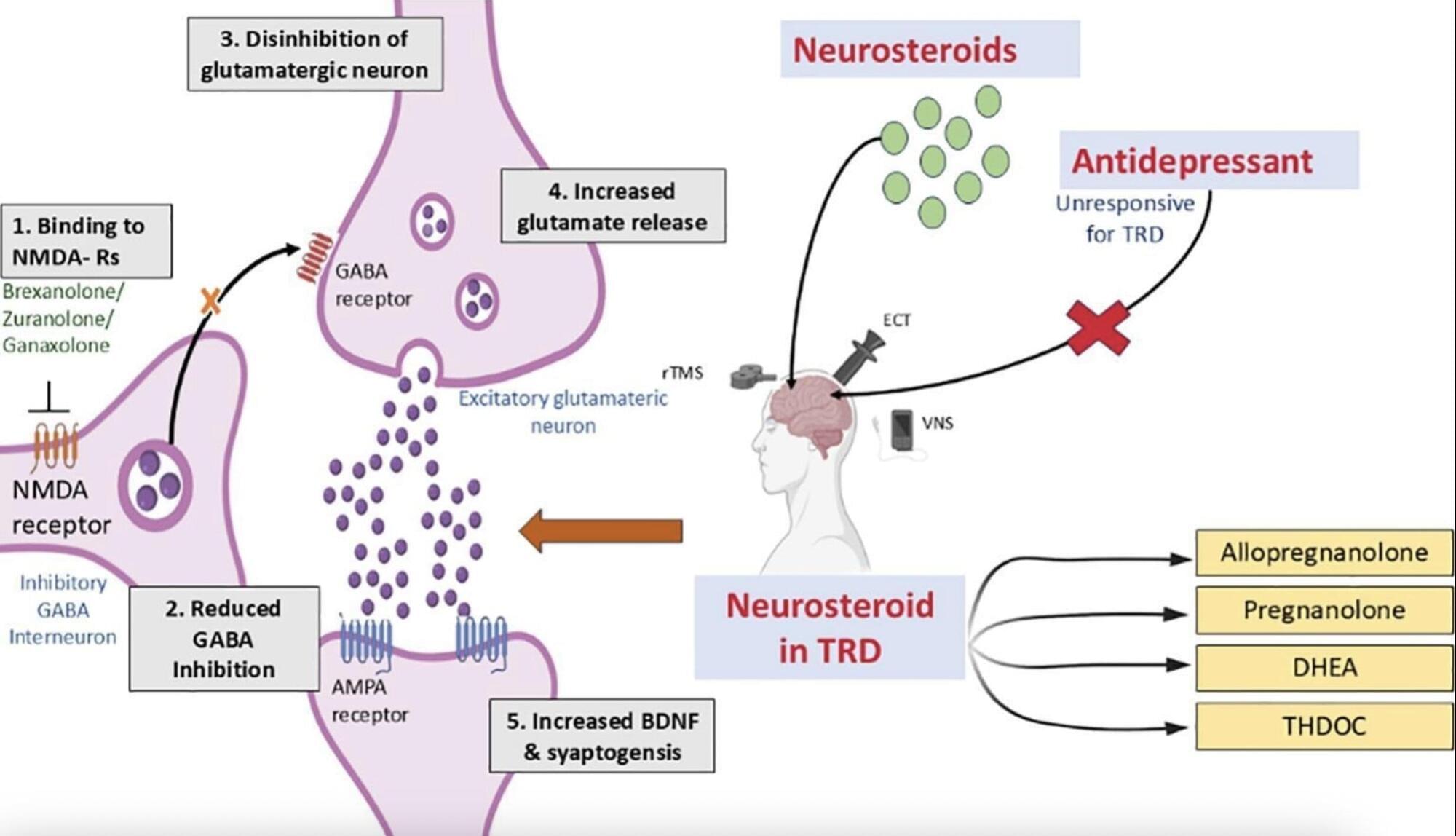
Depression, characterized by persistent sadness, hopelessness and a lack of interest in previously enjoyed activities, is one of the most common mental health disorders. Recent estimates by the World Health Organization (WHO) suggest that approximately 5% of the global population suffers from depression.
For decades, researchers have been trying to devise safe and effective treatments for depression that cause minimal or no side effects. This led to the introduction of a wide range of treatment strategies, ranging from psychotherapy and alternative medicine to a wide range of pharmacological drugs, including selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs) and atypical antidepressants.
Most people diagnosed with depression eventually find a suitable treatment for them via a trial-and-error process, ultimately leading to their recovery. Some individuals, however, can experience severe depression for prolonged periods of time, finding that no treatment ultimately eases their symptoms.