Facebook admitted something that should have been front-page news.
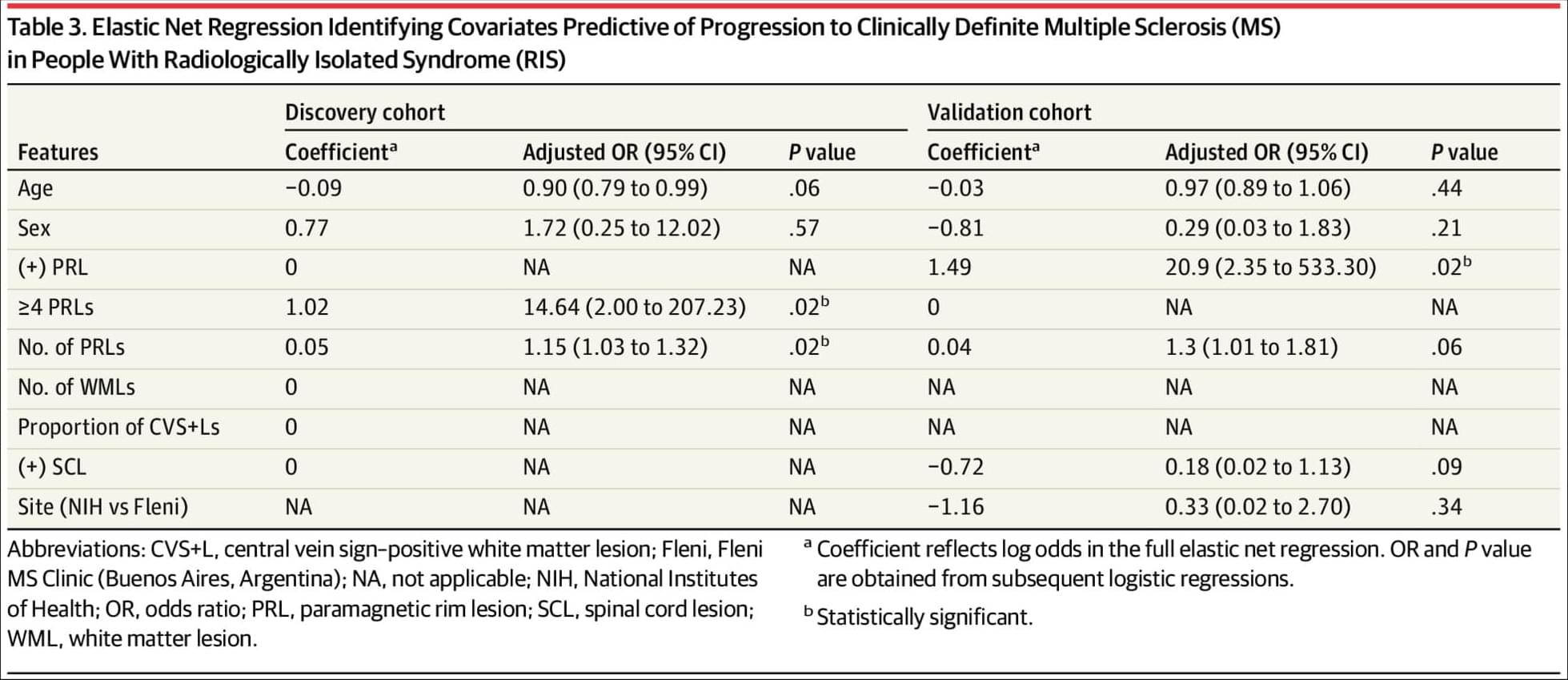
In an FTC antitrust filing, Meta revealed that only 7% of time on Instagram and 17% on Facebook is spent actually socializing with friends and family.
The rest?
Algorithmically selected content. Short-form video. Engagement optimized by AI.
This wasn’t a philosophical confession. It was a legal one. But it quietly confirms what many of us have felt for years:
What we still call “social networks” are not social.
They are attention machines.