Adopting a nanoscale approach to developing materials and designing experiments benefits research on batteries, supercapacitors and hybrid devices at all technology readiness levels.


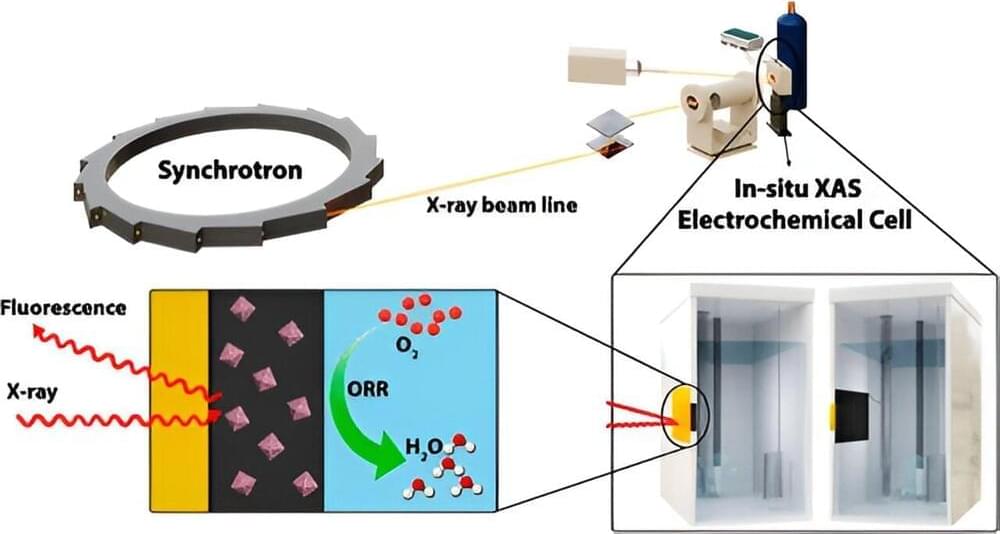
There is an urgent need to address climate change, making the development of sustainable energy alternatives more important than ever. While proton-exchange membrane fuel cells (PEMFCs) have shown great promise for energy production, particularly in the transportation industry, there is a long-standing problem with their durability and cost.
A Western research team has addressed the issue with a new cobalt-modified nanomaterial making PEMFCs more robust, readily sourced and environmentally sustainable demonstrating just a two percent loss in efficiency rate following 20,000 cycles in a durability test.
The new nanomaterial is used to enhance oxygen reduction reaction (ORR), the process that forms water in the fuel cell allowing a higher current for more efficient power generation. The cobalt-modified nanomaterial also reduces the reliance on platinum to construct these fuel cells. A costly precious metal, and mined primarily in South Africa, only a few hundred tons of platinum are produced annually.
VIEW NOW: https://ow.ly/GMX050PMTMH
In this webinar, three experts will discuss how Precision NanoSystems’ modular microfluidic platform technologies and analytics can help scientists successfully design, develop, test, and scale-up promising mRNA-LNP vaccines and therapeutics from concept to clinic. Don’t miss this webinar, now available on demand.
Nucleic acids (e.g., siRNA, mRNA and saRNA) can be designed and formulated to silence, express, and edit specific genes providing a flexible and powerful approach to preventing and treating diseases. The recent commercialization and widespread distribution of COVID-19 mRNA vaccines has exemplified the massive potential of this new class of genomic medicines and vaccines to effectively thwart emerging viral threats and treat a wide range of challenging diseases. Part of developing a successful mRNA therapeutic or vaccine is choosing a delivery mechanism that protects the nucleic acids on the way to their target tissue. Encapsulating mRNA in lipid nanoparticles has proven to be one of the best vehicles for overcoming extracellular and intracellular barriers and safely delivering the treatment. Several mRNA-LNP formulations that target things like viral infections and cancers are being evaluated clinically.
In this webinar, three experts will discuss how Precision Nanosystems’ modular microfluidic platform technologies and analytics can help scientists successfully design, develop, test, and scale-up promising mRNA-LNP vaccines and therapeutics from concept to clinic. They will provide an overview of Precision NanoSystem’s Biopharma Services, and share examples from internal R&D work that demonstrate the versatility of the genetic medicine toolbox for rapidly developing RNA-LNP vaccines. You’ll also learn about Precision NanoSystem’s BioAssay services and the capabilities that are available to facilitate and accelerate drug development projects.
In a move that echoes a sci-fi series, researchers have developed a super-small material that was able to not only stimulate nerves in rodents, but reconnect them as well. The finding could lead to injectable particles that take the place of larger implants.
In creating the particles, researchers at Rice University started with two layers of a metallic glass alloy called Metglas and wedged a piezoelectric layer of lead zirconium titanate in between them. Piezoelectric materials generate electricity when they have mechanical forces applied to them. Metglas is a magnetostrictive material, which means it changes its shape when it has a magnetic field applied to it. In this case, the change in shape of the Metglas in the presence of magnetic pulses caused the piezoelectric material inside to generate an electrical signal. Materials that do this are known as magnetoelectric.
“We asked, ‘Can we create a material that can be like dust or is so small that by placing just a sprinkle of it inside the body you’d be able to stimulate the brain or nervous system?’” said lead author Joshua Chen, a Rice doctoral alumnus. “With that question in mind, we thought that magnetoelectric materials were ideal candidates for use in neurostimulation. They respond to magnetic fields, which easily penetrate into the body, and convert them into electric fields – a language our nervous system already uses to relay information.”
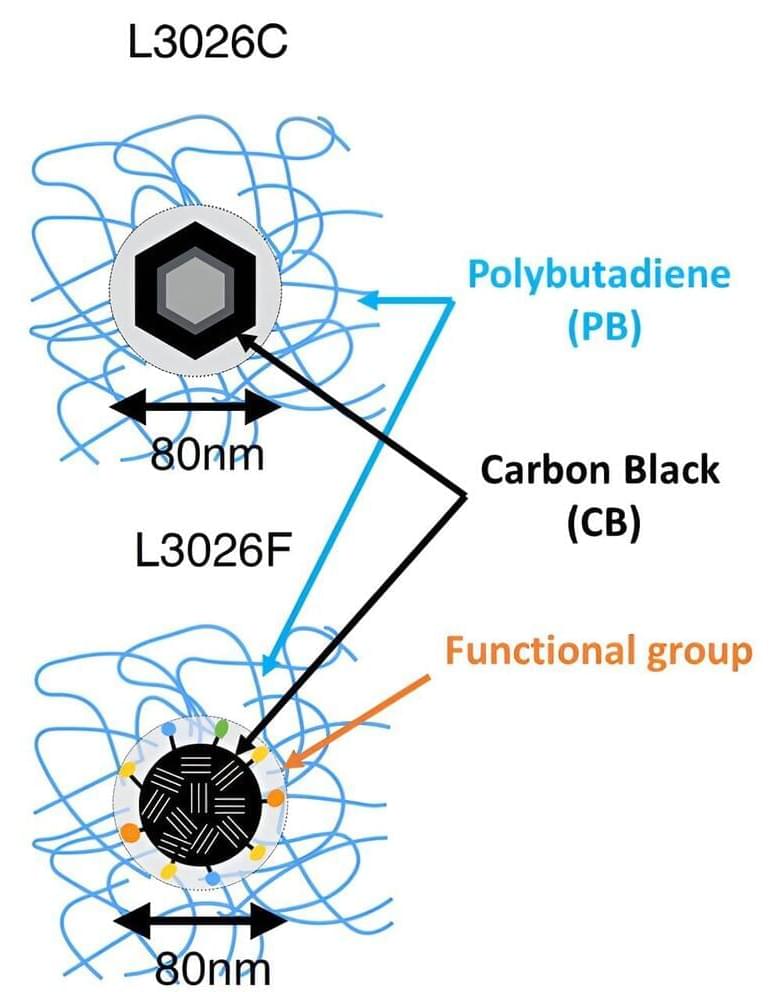
Scientists have observed the molecular motion of rubber components typically used in automobile tires—polybutadiene and carbon black—with the world’s fastest time resolution.
The study, published in Applied Physics Letters, reveals a clear interaction between the two components on the atomic scale, paving the way towards improved diagnostics of tire rubber degradation and the development of materials with enhanced durability.
Tire rubber is a composite material that typically includes synthetic rubber, such as polybutadiene, and added nanoparticles, such as carbon black, to improve its physical properties. During driving, strong forces act on the tire, causing its components to move against each another, which can lead to wear and degradation of the material.

Best known for co-discovering the gömböc—the first convex 3D shape with just two balancing points—Domokos aims to understand the physical world by describing its forms in the simplest possible geometry.
He often begins new projects by concocting original ways to classify shapes. To prove that the gömböc existed before they found it, he and Péter Várkonyi introduced mathematically precise definitions of flatness and thinness. To categorize pebbles, Domokos counts their number of stable and unstable balancing points. And to describe tessellating patterns in rock cracks or nanomaterials, he calculates just two numbers: the average number of “tiles” meeting at each vertex in the “mosaic” and the average number of vertices per tile.
The point is to find “a new language” to describe the shapes, says mathematician Krisztina Regős, one of Domokos’s graduate students. “The first thing that people do when they understand something: give it a name,” Domokos says. “And shapes don’t have names.”
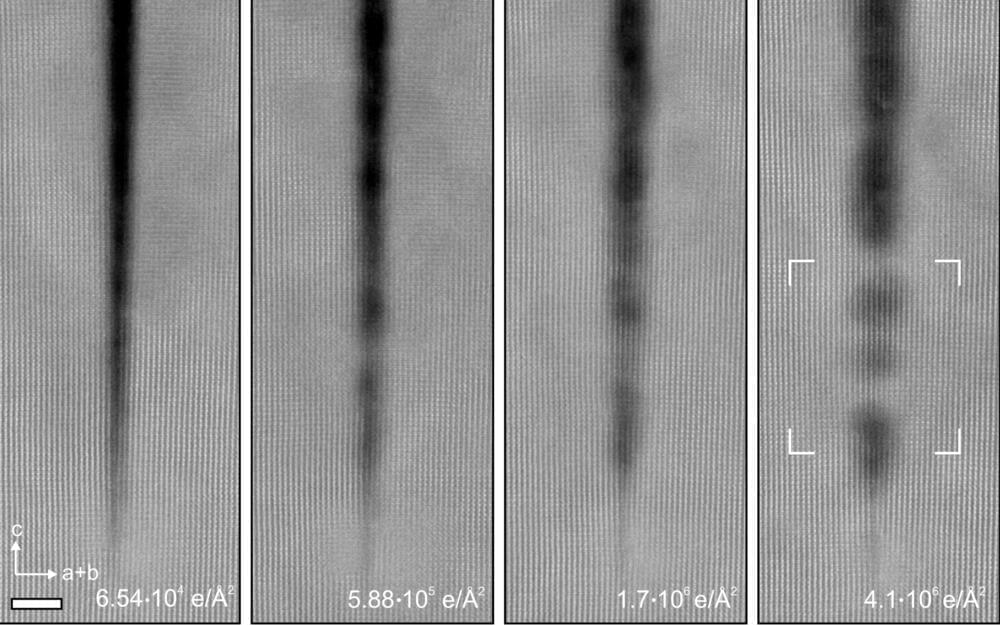
In a surprising new study, researchers at the University of Minnesota Twin Cities have found that the electron beam radiation that they previously thought degraded crystals can actually repair cracks in these nanostructures.
The groundbreaking discovery provides a new pathway to create more perfect crystal nanostructures, a process that is critical to improving the efficiency and cost-effectiveness of materials that are used in virtually all electronic devices we use every day.
“For a long time, researchers studying nanostructures were thinking that when we put the crystals under electron beam radiation to study them that they would degrade,” said Andre Mkhoyan, a University of Minnesota chemical engineering and materials science professor and lead researcher in the study. “What we showed in this study is that when we took a crystal of titanium dioxide and irradiate it with an electron beam, the naturally occurring narrow cracks actually filled in and healed themselves.”

Ceramic nanowires could essentially be used even for car tires reducing even hazardous rubber waste.
A team of MIT-led engineers found a simple, inexpensive way to strengthen Inconel 718 with ceramic nanowires to be used in metal PBF AM processes. The team believes that their general approach could be used to improve many other materials. “There is always a significant need for the development of more capable materials for extreme environments. We believe that this method has great potential for other materials in the future,” said Ju Li, the Battelle Energy Alliance Professor in Nuclear Engineering and a professor in MIT’s Department of Materials Science and Engineering (DMSE).
Li, who is also affiliated with the Materials Research Laboratory (MRL), is one of three corresponding authors of a paper on the work that appeared in the April 5 issue of Additive Manufacturing. The other corresponding authors are Professor Wen Chen of the University of Massachusetts at Amherst and Professor A. John Hart of the MIT Department of Mechanical Engineering.
Co-first authors of the paper are Emre Tekoğlu, an MIT postdoc in the Department of Nuclear Science and Engineering (NSE); Alexander D. O’Brien, an NSE graduate student; and Jian Liu of UMass Amherst. Additional authors are Baoming Wang, an MIT postdoc in DMSE; Sina Kavak of Istanbul Technical University; Yong Zhang, a research specialist at the MRL; So Yeon Kim, a DMSE graduate student; Shitong Wang, an NSE graduate student; and Duygu Agaogullari of Istanbul Technical University. The study was supported by Eni S.p. A. through the MIT Energy Initiative, the National Science Foundation, and ARPA-E.
When it comes to human longevity, you might envision nanobots helping our bodies operate more efficiently. But our bodies are biological machines in their own right, evolved to handle any situation in the real world from illness to cold to hunger. Our bodies heal themselves, and they can be programmed to do so if we understood that language better.
This video talks about DNA and genes, and the epigenetic mechanisms that read that information. The epigenetic clock is one way to measure the age of cells, and this can be reversed with current technologies. We discuss experiments by David Sinclair, which made blind mice see again, and experiments by Greg Fahy, which regenerated the immune system of humans and reset their cellular age by 2 years.
Asking our bodies to heal themselves could be one of the largest medical breakthroughs ever, instead of trying mainly chemical means of medication. And it has significant implications for whether or not we can achieve longevity escape velocity and continue to live more or less indefinitely. This promises to be a very interesting topic.
#aging #longevity #science.
The science of super longevity | Dr. Morgan Levine.
https://www.youtube.com/watch?v=B_CqKVU19ec.
Groundbreaking Research on Anti-Aging: Unlock the Secrets to Longevity | David Sinclair.
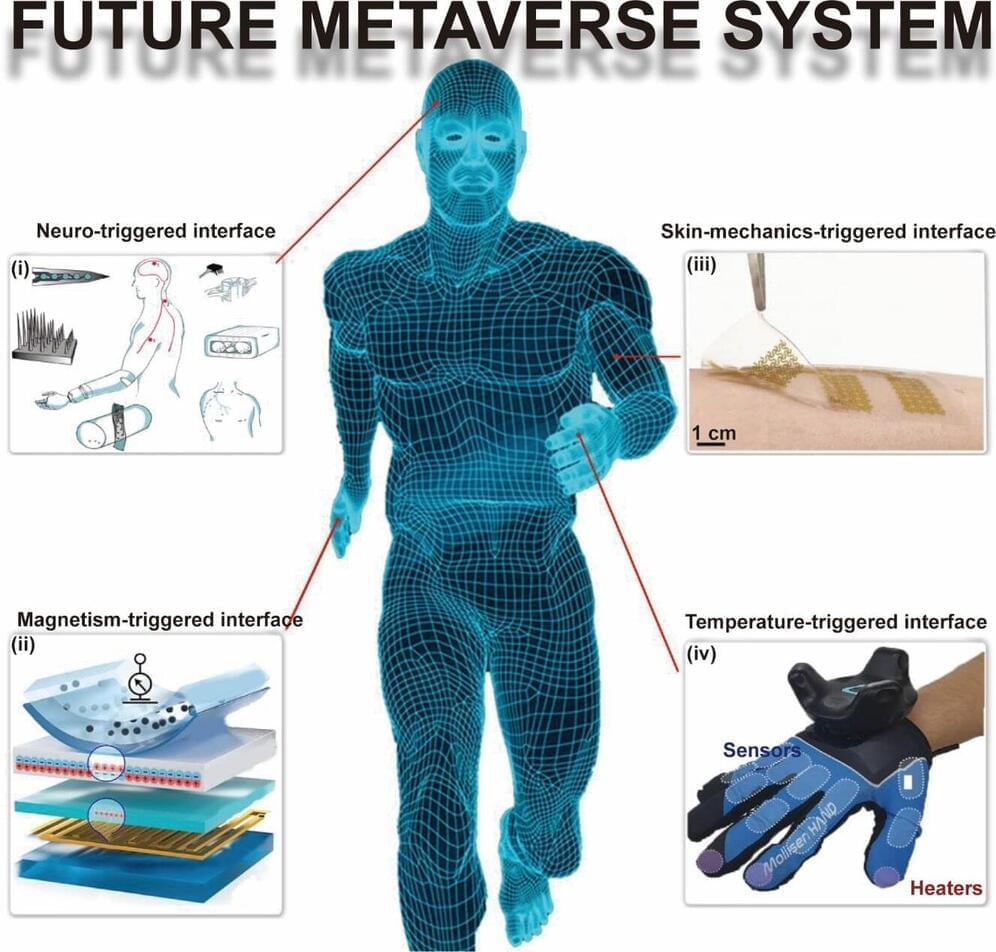
Researchers from Changchun University of Science and Technology (CUST) and City University of Hong Kong (CityU) have conducted a survey on the fabrication of flexible sensors using nanomaterials of different dimensions and the triggering methods of interaction between these sensors and virtual reality applications.
The review, published in the International Journal of Extreme Manufacturing (IJEM), highlights the recent advancements in nanomaterial-based flexible sensors (NMFSs) involving various nanomaterial frameworks such as nanoparticles, nanowires, and nanofilms.
Different triggering mechanisms for interaction between NMFSs and metaverse/virtual reality applications are discussed, e.g., skin-mechanics-triggered, temperature-triggered, magnetically triggered, and neural-triggered interfaces.