This is a detailed summary of plasma dilution and at 58:38 the future is explained where they will publish human results from 25 people, then start a company whose first order of business will be phase 3 trials with more people and placebo and hopefully funding. It appears you can pay to have the procedure. The hopeful start is this year in may.
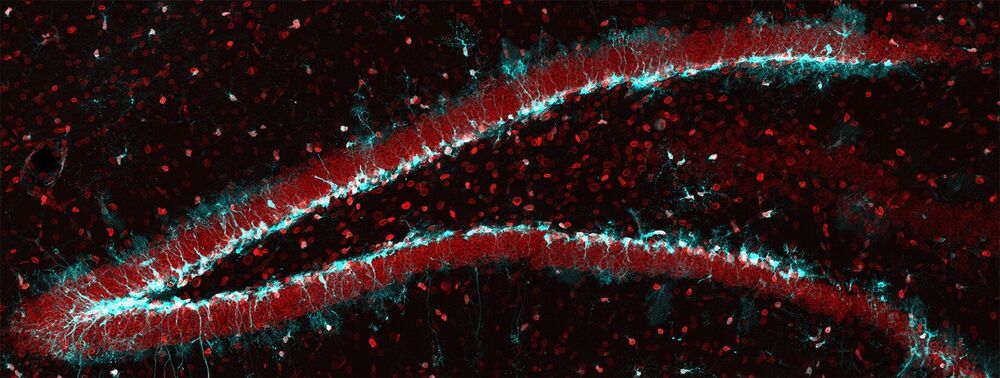
Irina will present her recent findings on plasma dilution, showing that age-reversing effects, such as rejuvenating tissues in mice, can be achieved by.
diluting the blood plasma of old mice: Rejuvenation of three germ layers tissues by exchanging old blood plasma with saline-albumin.
Irina’s research focus.
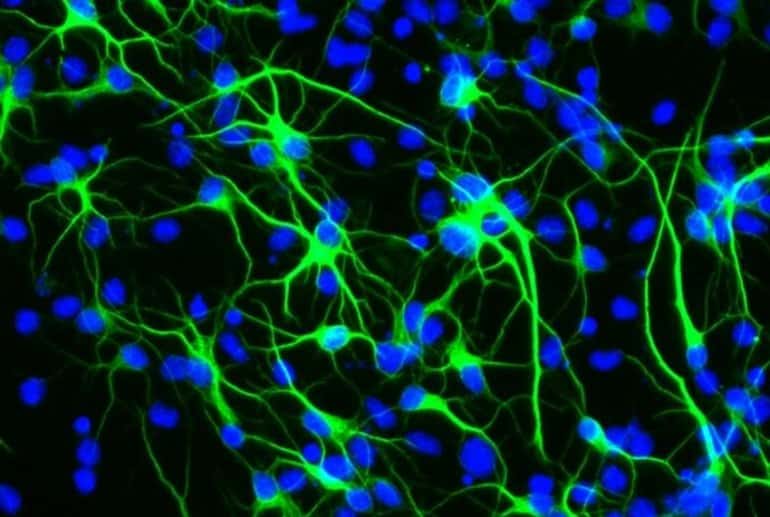
A key direction of my laboratory is to understand age-imposed and pathological changes in molecular compositions of systemic and local environments of adult stem cells and to calibrate these to health — youth. In the past few years this direction has been ramified into synthetic biology, CRISPR technologies, bio-orthogonal proteomics and development of innovative digital bio-sensors that we collaboratively applied to the fields of aging and diagnostics of genetic diseases. Success in this research will improve our understanding of the determinants of homeostatic health and will enable novel rational approaches to treat a number of degenerative, fibrotic, metabolic and inflammatory diseases, as a class.
Zoom Transcription:
https://otter.ai/u/yhmNLEM7V52oOfW93lUfDWqL_uw