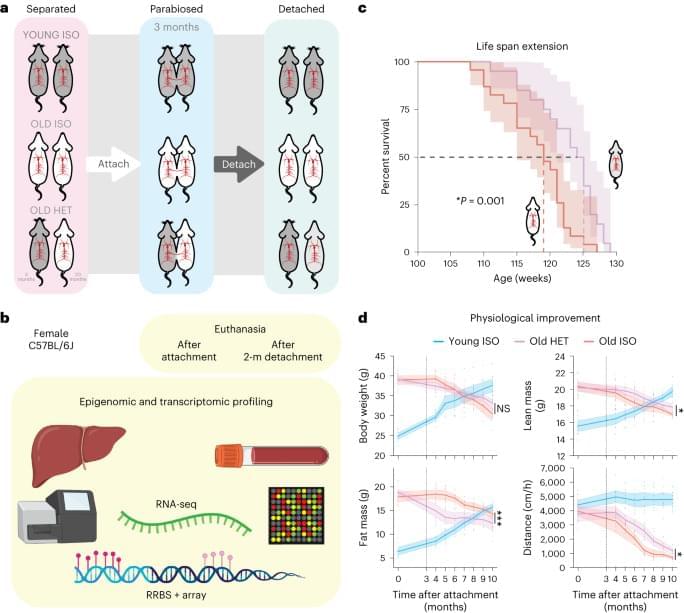
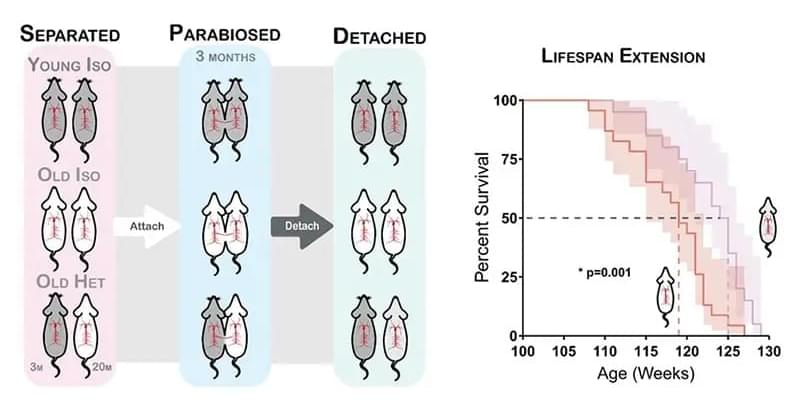
The skin is the largest organ of the body, comprising several compartments and about 20 different cell types that are involved in various skin functions – complexity that is more than skin deep! [1] Skin aging is a multifactorial process that is impacted by several intrinsic and extrinsic factors. Constant exposure of the human skin to such stimuli impacts its function and accelerates aging resulting in dry skin, wrinkling, thinning of the epidermis, and reduced barrier integrity. While we notice most of those changes by looking in a mirror – something the cosmetics industry has leveraged to multi-billion dollar effect – older skin is more at risk of injury, less able to sense touch, heat and cold, slower to heal and more prone to cellulitis and other skin infections.
Aging is a complex and gradual process characterized by a reduction in function and reproducibility along with an increase in the incidence of degenerative diseases. Skin aging has been reported to be associated with the presence and accumulation of senescent cells. “A number of diseases that increase in older people may have a unifying underlying mechanism having to do with senescence,” says Ruth Montgomery, PhD, professor of medicine and epidemiology (microbial diseases) at Yale School of Medicine [2].
Senescent cells are those that have lost their proliferative capacity, are resistant to apoptosis, and secrete factors that can cause tissue deterioration and inflammation [3]. These factors are termed senescence-associated secretory phenotype (SASP) and can lead to extracellular matrix (ECM) deposition, impact epidermal stem cell renewal and worsen melanin synthesis.