This is a ~50 minute talk by Michael Levin to a clinical audience about bioelectricity and why it represents a new approach to medicine.
Category: life extension – Page 188

Genflow makes progress towards clinical trial of NASH gene therapy
Gene therapy company Genflow Biosciences has received positive feedback from Belgium’s Federal Agency for Medicines and Health Products as it seeks to move into human clinical trials. Genflow is developing gene therapies that target the aging process, with a focus on reducing and delaying age-related diseases.
Genflow’s approach involves the use of adeno-associated virus (AAV) vectors to deliver copies of the Sirtuin-6 (SIRT6) gene variant found in centenarians into cells. Sirtuins are a group of proteins that play a vital role in regulating various cellular processes. In recent years, SIRT6 has gained attention for its potential role in promoting healthy aging.
Genflow says it has received written advice from the FAHMP to commence clinical trials of its lead compound (GF-1002) in patients suffering from NASH, an aggressive form of non-alcoholic fatty liver disease, rather than in healthy volunteers. While further discussions and agreement with the European Medicine Agency (EMA) are still required, Genflow says that it expects a NASH clinical trial to commence in approximately 18 months.
Longevity Summit Dublin
The last 2 questions and the answers are great. The first starts at 30 minutes. And I like his answer to the 2nd question especially, the time is 33:54. “What is giving me great hope is that we’re entering the phases where we have more than enough tools to get really get close to escape velocity.”
Genome Engineering for Healthy Longevity – George Church at Longevity Summit Dublin 2023.
#GeorgeChurch #GenomeEngineering #HealthyLongevity #LongevitySummitDublin2023 #AgingResearch #DublinConference #LongevityScience #BiomedicalEngineering #GeneticModification #DublinTalks #GenomicInnovation #MedicalScience #LongevityResearch #PrecisionMedicine #AgingInterventions #Healthspan #GenomeEditing #AntiAging #LongevityInsights #Genetics #Innovation

Insilico uses Microsoft’s BioGPT to find targets for aging and disease
Insilico Medicine, a clinical-stage generative AI-driven drug discovery company has announced that the company has used Microsoft BioGPT to identify targets against both the aging process and major age-related diseases.
Longevity. Technology: ChatGPT – the AI chatbot – can craft poems, write webcode and plan holidays. Large language models (LLMs) are the cornerstone of chatbots like GPT-4; trained on vast amounts of text data, they have been contributing to advances in diverse fields including literature, art and science – but their potential in the complex realms of biology and genomics has yet to be fully unlocked.
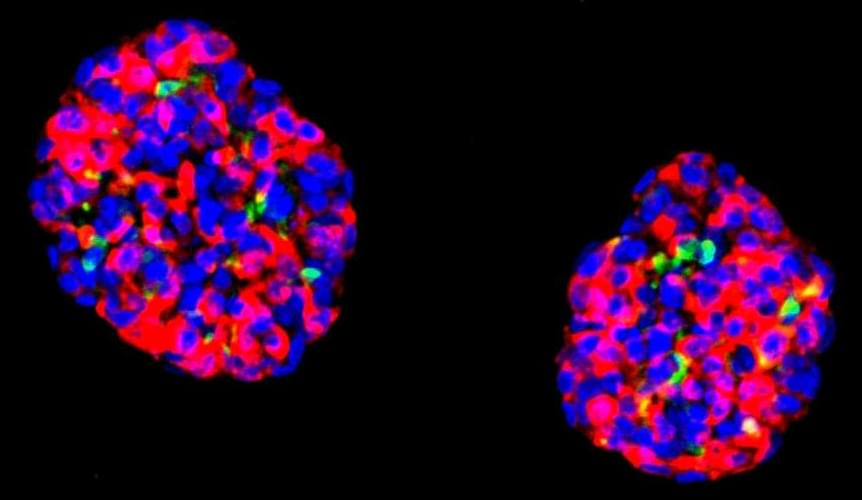
Scientists Target Human Stomach Cells for Diabetes Therapy
Stem cells from the human stomach can be converted into cells that secrete insulin in response to rising blood sugar levels, offering a promising approach to treating diabetes, according to a preclinical study from researchers at Weill Cornell Medicine.
In the study, which appeared April 27 in Nature Cell Biology, the researchers showed that they could take stem cells obtained from human stomach tissue and reprogram them directly—with strikingly high efficiency—into cells that closely resemble pancreatic insulin-secreting cells known as beta cells. Transplants of small groups of these cells reversed disease signs in a mouse model of diabetes.
“This is a proof-of-concept study that gives us a solid foundation for developing a treatment, based on patients’ own cells, for type 1 diabetes and severe type 2 diabetes,” said study senior author Dr. Joe Zhou, an associate professor of regenerative medicine and a member of the Hartman Institute for Therapeutic Organ Regeneration at Weill Cornell Medicine.
Longevity: can ageing be reversed?
Ageing has always been inevitable but fasting, epigenetic reprogramming and parabiosis are just some of the scientific techniques that seem to help people stay young. Might the Peter Pan dream become real?
00:00 — Can science turn back the clock?
01:01 — Centenarians.
02:51 — What is ageing?
04:51 — Dietary restriction.
06:00 — Roundworms.
07:55 — Epigenetics.
09:43 — Blood and guts.
11:40 — Senolytics.
12:38 — Metformin.
13:51 — Anti-ageing treatments are coming.
Sign up to The Economist’s daily newsletter: https://econ.st/3QAawvI
Read the Technology Quarterly on longevity: https://econ.st/462fqto.
Christian Californians may have a solution to America’s obesity: https://econ.st/3EC4GG9
How to eat to 100: https://econ.st/3EwQTAq.
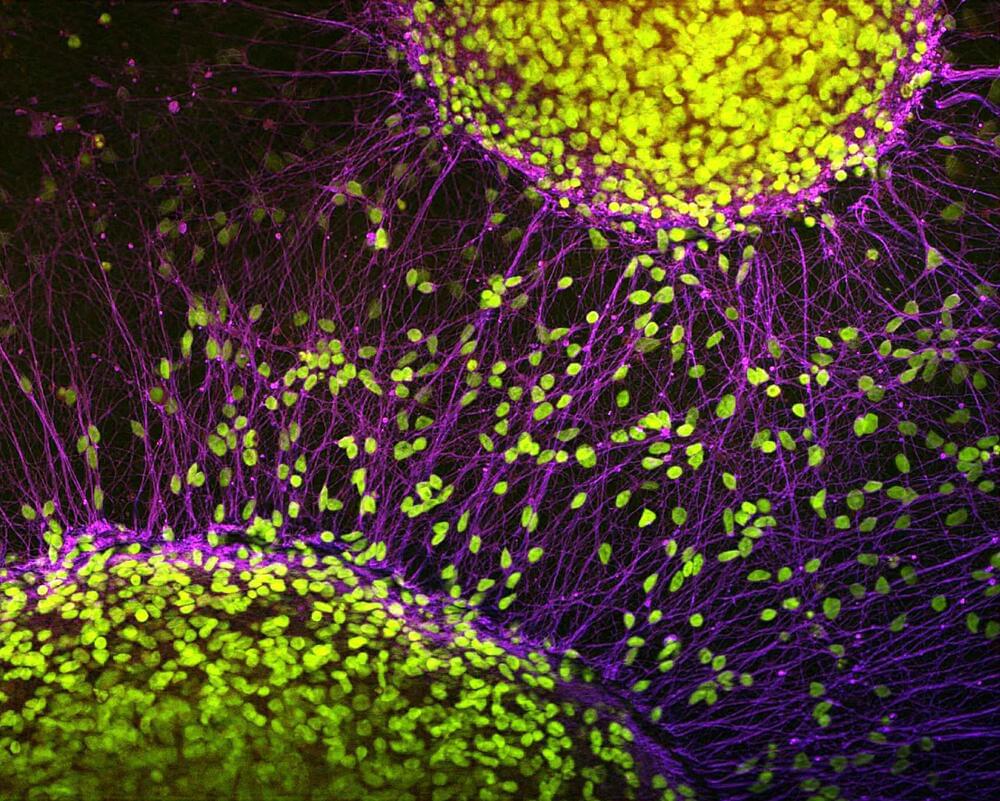
New method tracks how brain cells age
Hospital nurseries routinely place soft bands around the tiny wrists of newborns that hold important identifying information such as name, sex, mother, and birth date. Researchers at Rockefeller University are taking the same approach with newborn brain cells—but these neonates will keep their ID tags for life, so that scientists can track how they grow and mature, as a means for better understanding the brain’s aging process.
As described in a new paper in Cell, the new method developed by Rockefeller geneticist Junyue Cao and his colleagues is called TrackerSci (pronounced “sky”). This low-cost, high-throughput approach has already revealed that while newborn cells continue to be produced through life, the kinds of cells being produced greatly vary in different ages. This groundbreaking work, led by co-first authors Ziyu Lu and Melissa Zhang from Cao’s lab, promises to influence not only the study of the brain but also broader aspects of aging and disease across the human body.
“The cell is the basic functional unit of our body, so changes to the cell essentially underlie virtually every disease and the aging process,” says Cao, head of the Laboratory of Single-Cell Genomics and Population Dynamics. “If we can systematically characterize the different cells and their dynamics using this novel technique, we may get a panoramic view of the mechanisms of many diseases and the enigma of aging.”

Omega-3 fatty acids may slow age related hearing loss
Hearing diminishes as we age — about 50% of adults 75 and over in the United States have disabling hearing loss.
Age-related hearing loss cannot currently be stopped.
Researchers from the University of Guelph and Tufts.
University/Fatty Acid Research Institute have found a link between increased omega-3 fatty acids in the blood and less age-related hearing issues.
As we age, it is not uncommon for the effectiveness of some of our sensesTrusted Source — including vision, hearing, and tasteTrusted Source — to decrease.
In fact, research shows the rate of hearing loss increases with ageTrusted Source. In the United States, about 25% of people ages 65 to 74 and almost half of adults aged 75 and… More.

Individuals prone to antisocial behavior age faster, study finds
An analysis of data from the Dunedin Multidisciplinary Health and Development study, a large longitudinal study in New Zealand, showed that participants with a history of antisocial behavior had a significantly faster pace of biological aging. When these individuals reached the calendar age of 45, they were on average 4.3 years older biologically compared to those who had lower levels of antisocial behavior. The study was published in the International Journal of Environmental Research and Public Health.
Antisocial behavior refers to actions that consistently violate social norms, disregard the rights of others, and often involve a lack of empathy or remorse. It involves behaviors such as deceitfulness, aggression, theft, violence, lying, and other behaviors that are harmful, manipulative, or exploitative towards others.
Antisocial behavior is typically associated with youth. This type of behavior starts between the ages of 8 and 14, peaks between 15 and 19, and usually becomes less frequent between the ages of 20 and 29. Although it becomes less common with age, it seems to have a lasting negative impact on health. Studies have shown that individuals who exhibit antisocial behaviors in their youth tend to have worse health outcomes as adults compared to their peers.
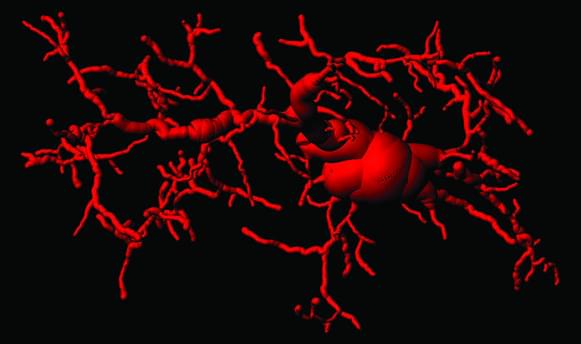
Signaling Pathway Implicated in Inflammation and Functional Decline during Aging
Low-grade inflammation contributes to age-related decline and impairment, but the precise pathways responsible for this inflammation and their impact on natural aging have until now remained elusive.
A study headed by researchers at the Swiss Federal Institute of Technology Lausanne (EPFL) has now shown that a molecular signaling pathway known as cGAS/STING plays a critical role in driving chronic inflammation and functional decline during aging. Andrea Ablasser, PhD, and colleagues found that blocking the STING protein suppressed inflammatory responses in human senescent cells and tissues, and reduced aging-related inflammation in multiple peripheral organs and in the brain in mice.
The researchers in addition studied the effects of blocking the STING protein in aged mice. As expected by its central role in driving inflammation, inhibiting STING alleviated markers of inflammation both in the periphery and in the brain. “Notably, various aging-related immune signature genes were significantly attenuated as a result of STING inhibition,” they stated. And importantly, animals receiving STING inhibitors displayed significant enhancements in spatial and associative memory, as well as improved muscle strength and physical endurance.
“Consistently, STING inhibition by H-151, a brain permeable compound, reduced the levels of immune-related signature genes in the brains of aged mice,” the scientists pointed out. “Together, these results establish STING as an important driver of aging-associated inflammation, both in the periphery and the CNS, promoting frailty and cognitive decline.”
The study advances our understanding of aging-related inflammation and also offers potential strategies for slowing cognitive deterioration in age-associated neurodegenerative conditions. The precise elucidation of the neuroimmune crosstalk governing microglial-dependent neurotoxicity also holds promise for the future study of neurodegenerative diseases. The team concluded, “Together with previous studies in models of Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis and frontotemporal dementia, and Nieman–Pick’s disease, our study reveals notable convergence on cGAS–STING signaling in chronic neurodegenerative conditions … Our findings establish the cGAS–STING pathway as a driver of aging-related inflammation in peripheral organs and the brain, and reveal blockade of cGAS–STING signaling as a potential strategy to halt neurodegenerative processes during old age.”