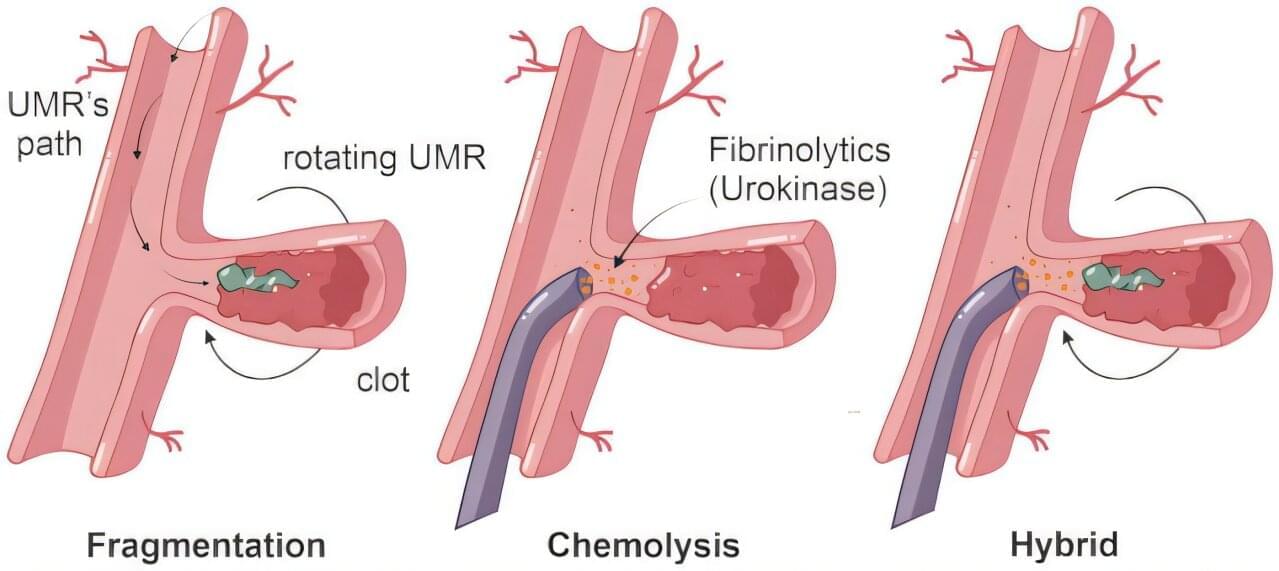
Researchers at the TechMed Center of the University of Twente and Radboud University Medical Center have removed blood clots with wireless magnetic robots. This innovation promises to transform treatment for life-threatening vascular conditions like thrombosis.
Cardiovascular diseases such as thrombosis are a major global health challenge. Each year worldwide, 1 in 4 people die from conditions caused by blood clots. A blood clot blocks a blood vessel, preventing the blood from delivering oxygen to certain areas of the body.
Minimally invasive Traditional treatments struggle with clots in hard-to-reach areas. But magnetic microrobots bring hope to patients with otherwise inoperable clots. The screw-shaped robots can navigate through intricate vascular networks since they are operated wirelessly.