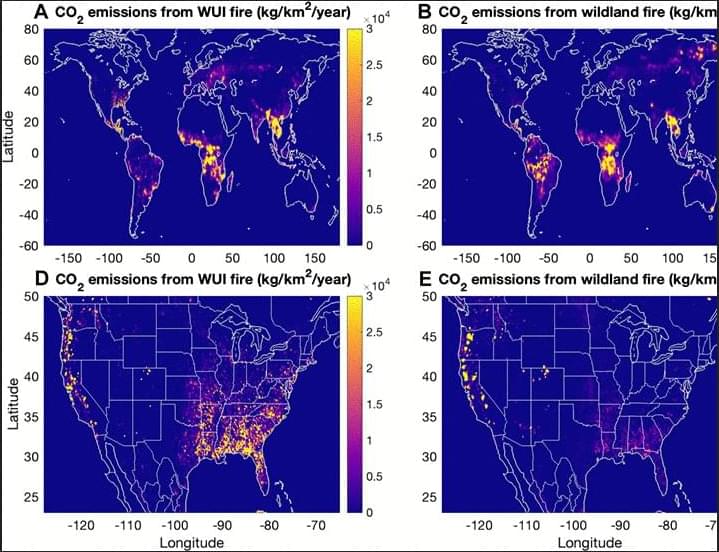
Proximity of WUI fires leads to disproportionately large impacts on air quality and human health especially for urban population.


A drug-resistant type of bacteria that has adapted to health care settings evolved in the past several years to weaponize an antimicrobial genetic tool, eliminating its cousins and replacing them as the dominant strain. University of Pittsburgh School of Medicine scientists made the discovery when combing through local hospital data—and then confirmed that it was a global phenomenon.
The finding, published in Nature Microbiology, may be the impetus for new approaches in developing therapeutics against some of the world’s deadliest bacteria. It also validates a new use for a system developed at Pitt and UPMC that couples genomic sequencing with computer algorithms to rapidly detect infectious disease outbreaks.
“Our lab has a front row seat to the parade of pathogens that move through the hospital setting,” said senior author Daria Van Tyne, Ph.D., associate professor of medicine in Pitt’s Division of Infectious Diseases. “And when we took a step back and zoomed out, it quickly became apparent that big changes were afoot with one of the world’s more difficult-to-treat bacteria.”


Additional experiments revealed that mice given access to an exercise wheel or treated with antidepressants also exhibited increased CB1 receptor levels in astrocytes. Furthermore, analysis of human brain tissue from the Douglas-Bell Canada Brain Bank indicated that individuals with major depression had lower astrocytic CB1 receptor levels compared to those without depression or those who had received antidepressant treatment.
Implications for mental health interventions
These findings raise the possibility of developing treatments that selectively activate CB1 receptors in astrocytes to mitigate anxiety and depression. However, the challenge remains in limiting activation to astrocytes, as prolonged CB1 receptor stimulation in neurons can lead to side effects affecting alertness, anxiety and appetite. Until targeted pharmacological interventions become available, engaging in physical activity may help protect against stress-related mental health conditions by enhancing CB1 receptor expression.
A new study by UCLA Health has discovered what researchers say is the first drug to fully reproduce the effects of physical stroke rehabilitation in model mice, following from human studies.
The findings, published in Nature Communications, tested two candidate drugs derived from their studies on the mechanism of the brain effects of rehabilitation, of which one resulted in significant recovery in movement control after stroke in the mouse model.
Stroke is the leading cause of adult disability because most patients do not fully recover from the effects of stroke. There are no drugs in the field of stroke recovery, requiring stroke patients to undergo physical rehabilitation which has shown to be only modestly effective.

A study from Tübingen University and the German Center for Diabetes Research reveals that the brain plays a crucial role in obesity and type 2 diabetes development. It shows that even a brief period of consuming high-calorie processed foods can significantly alter brain insulin sensitivity, a key factor in weight gain and metabolic disorders. The research demonstrated that insulin’s appetite-suppressing effect in the brain diminishes after a short-term high-calorie diet, leading to insulin resistance. These effects were observed in healthy participants, suggesting that dietary habits could influence brain function before any significant weight gain occurs. Further research is needed to understand the brain’s role in these conditions.
The number of obese persons has grown significantly in recent decades, which presents significant difficulties for those who are impacted, healthcare systems, and those who provide treatment. The hormone insulin plays a key role in the development of obesity. Up until recently, there have been numerous signs indicating insulin causes neurodegenerative and metabolic disorders, especially in the brain. A recent study by the University Hospital of Tübingen, the German Center for Diabetes Research (DZD), and Helmholtz Munich offers intriguing new insights into the origins of type 2 diabetes and obesity as well as the brain’s function as a critical control center.
Obesity has only been officially recognized as a disease in Germany since 2020, despite the fact that it has long been known to cause a number of illnesses, including diabetes, heart attacks, and even cancer. The World Health Organization has already declared obesity to be an epidemic, affecting over one billion individuals globally and almost 16 million in Germany alone. A body mass index of 30 or more is considered obese, and a poor diet and insufficient exercise are frequently cited as the causes of this chronic illness. However, the mechanisms in the body that lead to obesity and cause the disease are more complex.
Obesity and the role of insulin in the brain
Unhealthy body fat distribution and chronic weight gain are linked to the brain’s sensitivity to insulin. What specific functions does insulin perform in the brain, and how does it affect individuals of normal weight? In their study, Prof. Dr. Stephanie Kullmann and her colleagues at the Tübingen University Hospital for Diabetology, Endocrinology, and Nephrology found the answer to this query. “Our findings demonstrate for the first time that even a brief consumption of highly processed, unhealthy foods (such as chocolate bars and potato chips) causes a significant alteration in the brain of healthy individuals, which may be the initial cause of obesity and type 2 diabetes,” says Prof. Kullmann, the study’s leader. In a healthy state, insulin has an appetite-suppressing effect in the brain. However, in people with obesity in particular, insulin no longer regulates eating behavior properly, resulting in insulin resistance.

How does the armored tiling on shark and ray cartilage maintain a continuous covering as the animals’ skeletons expand during growth?
This is a question that has perplexed Professor Mason Dean, a marine biologist in the Department of Infectious Diseases and Public Health at City University of Hong Kong (CityUHK) since he was in graduate school.
An expert in skeletal development, structure and function in vertebrate animals, but with a particular focus on (and affection for) sharks and rays, Professor Dean says he was curious about how nature keeps complex surfaces covered while organs and animals are growing, and their surfaces are changing.

Snap a photo of your meal, and artificial intelligence instantly tells you its calorie count, fat content, and nutritional value—no more food diaries or guesswork.
This futuristic scenario is now much closer to reality, thanks to an AI system developed by NYU Tandon School of Engineering researchers that promises a new tool for the millions of people who want to manage their weight, diabetes and other diet-related health conditions.
The technology, detailed in a paper presented at the 6th IEEE International Conference on Mobile Computing and Sustainable Informatics, uses advanced deep-learning algorithms to recognize food items in images and calculate their nutritional content, including calories, protein, carbohydrates and fat.

Brain implants hold immense promise for restoring function in patients with paralysis, epilepsy and other neurological disorders. But a team of researchers at Case Western Reserve University has discovered that bacteria can invade the brain after a medical device is implanted, contributing to inflammation and reducing the device’s long-term effectiveness.
The research, published in Nature Communications, could improve the long-term success of brain implants now that a target has been identified to address.
“Understanding the role of bacteria in implant performance and brain health could revolutionize how these devices are designed and maintained,” said Jeff Capadona, Case Western Reserve’s vice provost for innovation, the Donnell Institute Professor of Biomedical Engineering and senior research career scientist at the Louis Stokes Cleveland VA Medical Center.
Science, Policy And Advocacy For Impactful And Sustainable Health Ecosystems — Dr. Catharine Young, Ph.D. — fmr. Assistant Director of Cancer Moonshot Policy and International Engagement, White House Office of Science and Technology Policy (OSTP)
Dr. Catharine Young, Ph.D. recently served as Assistant Director of Cancer Moonshot Policy and International Engagement at the White House Office of Science and Technology Policy (https://www.whitehouse.gov/ostp/) where she served at OSTP to advance the Cancer Moonshot (https://www.cancer.gov/research/key-i… with a mission to decrease the number of cancer deaths by 50% over the next 25 years.
Dr. Young’s varied career has spanned a variety of sectors including academia, non-profit, biotech, and foreign government, all with a focus on advancing science.
Dr. Young previously served as Executive Director of the SHEPHERD Foundation, where she championed rare cancer research and drove critical policy changes. Her work has also included fostering interdisciplinary collaborations and advancing the use of AI, data sharing, and clinical trial reform to accelerate cancer breakthroughs.
Dr. Young’s leadership in diplomacy and innovation includes roles such as Senior Director of Science Policy at the Biden Cancer Initiative and Senior Science and Innovation Policy Advisor at the British Embassy, where she facilitated international agreements to enhance research collaborations.