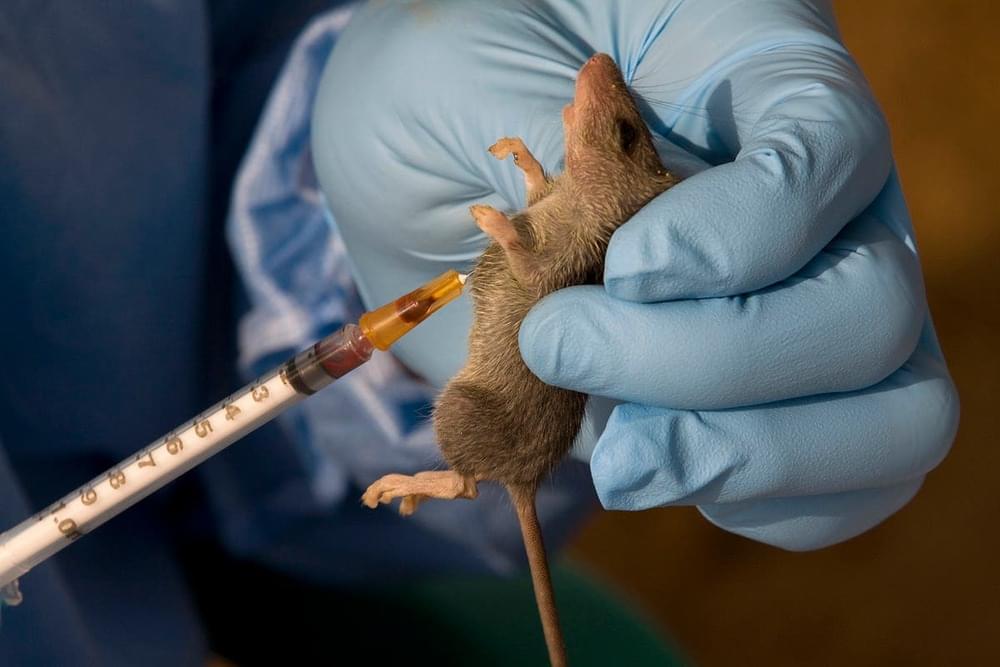
Lassa fever is like ebola and there is an outbreak in Nigeria. It is transmissible through inhalation.
The death of a patient in the UK suffering from Lassa fever has heightened concern around the illness after a third case was reported.
The UK Health Security Agency (UKHSA) said it was contacting individuals who had been in close contact with the infected patients after the death was confirmed last week.
Commenting on the recent cases detected in the UK, which were linked to travel to west Africa, Dr Susan Hopkins, chief medical advisor at UKHSA, said: Cases of Lassa fever are rare in the UK and it does not spread easily between people.