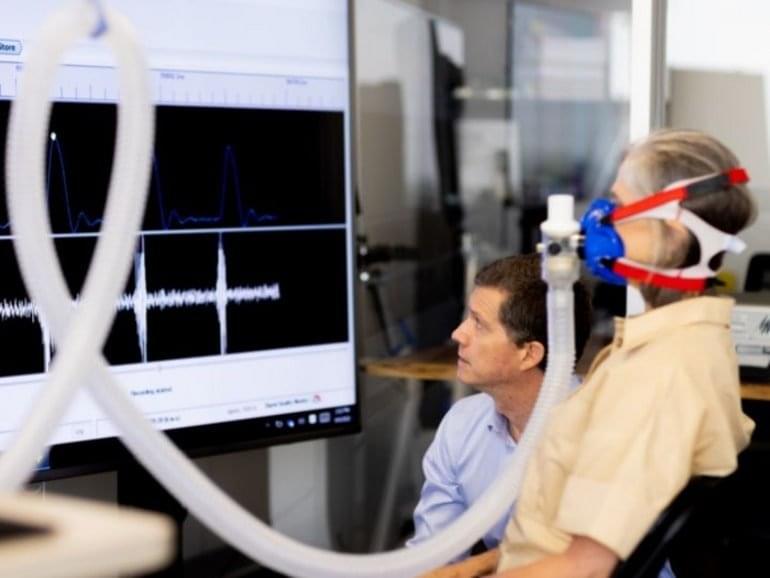
Neurodegenerative diseases—like amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease), Alzheimer’s, and Parkinson’s—are complicated, chronic ailments that can present with a variety of symptoms, worsen at different rates, and have many underlying genetic and environmental causes, some of which are unknown. ALS, in particular, affects voluntary muscle movement and is always fatal, but while most people survive for only a few years after diagnosis, others live with the disease for decades. Manifestations of ALS can also vary significantly; often slower disease development correlates with onset in the limbs and affecting fine motor skills, while the more serious, bulbar ALS impacts swallowing, speaking, breathing, and mobility. Therefore, understanding the progression of diseases like ALS is critical to enrollment in clinical trials, analysis of potential interventions, and discovery of root causes.
However, assessing disease evolution is far from straightforward. Current clinical studies typically assume that health declines on a downward linear trajectory on a symptom rating scale, and use these linear models to evaluate whether drugs are slowing disease progression. However, data indicate that ALS often follows nonlinear trajectories, with periods where symptoms are stable alternating with periods when they are rapidly changing. Since data can be sparse, and health assessments often rely on subjective rating metrics measured at uneven time intervals, comparisons across patient populations are difficult. These heterogenous data and progression, in turn, complicate analyses of invention effectiveness and potentially mask disease origin.
Now, a new machine-learning method developed by researchers from MIT, IBM Research, and elsewhere aims to better characterize ALS disease progression patterns to inform clinical trial design.