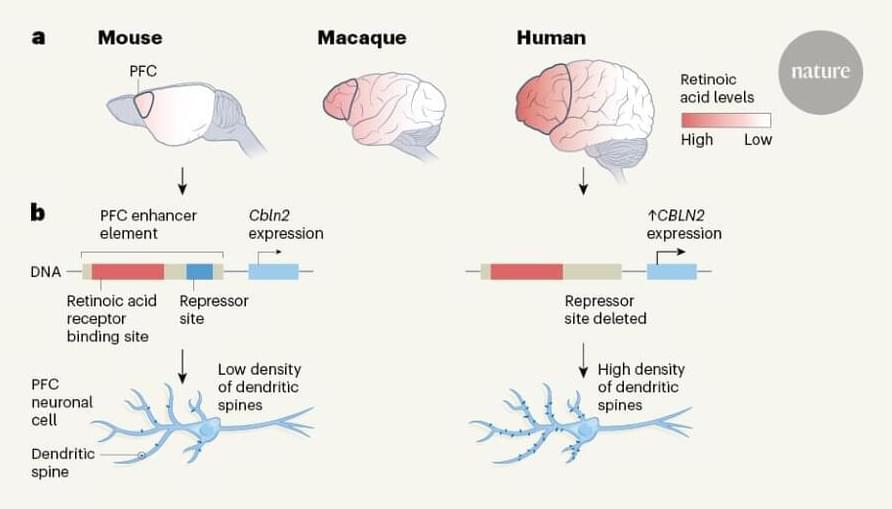
Based on Transformers, our new architecture advances genetic research by improving the ability to predict how DNA sequence influences gene expression.
When the Human Genome Project succeeded in mapping the DNA sequence of the human genome, the international research community were excited by the opportunity to better understand the genetic instructions that influence human health and development. DNA carries the genetic information that determines everything from eye colour to susceptibility to certain diseases and disorders. The roughly 20,000 sections of DNA in the human body known as genes contain instructions about the amino acid sequence of proteins, which perform numerous essential functions in our cells. Yet these genes make up less than 2% of the genome. The remaining base pairs — which account for 98% of the 3 billion “letters” in the genome — are called “non-coding” and contain less well-understood instructions about when and where genes should be produced or expressed in the human body.