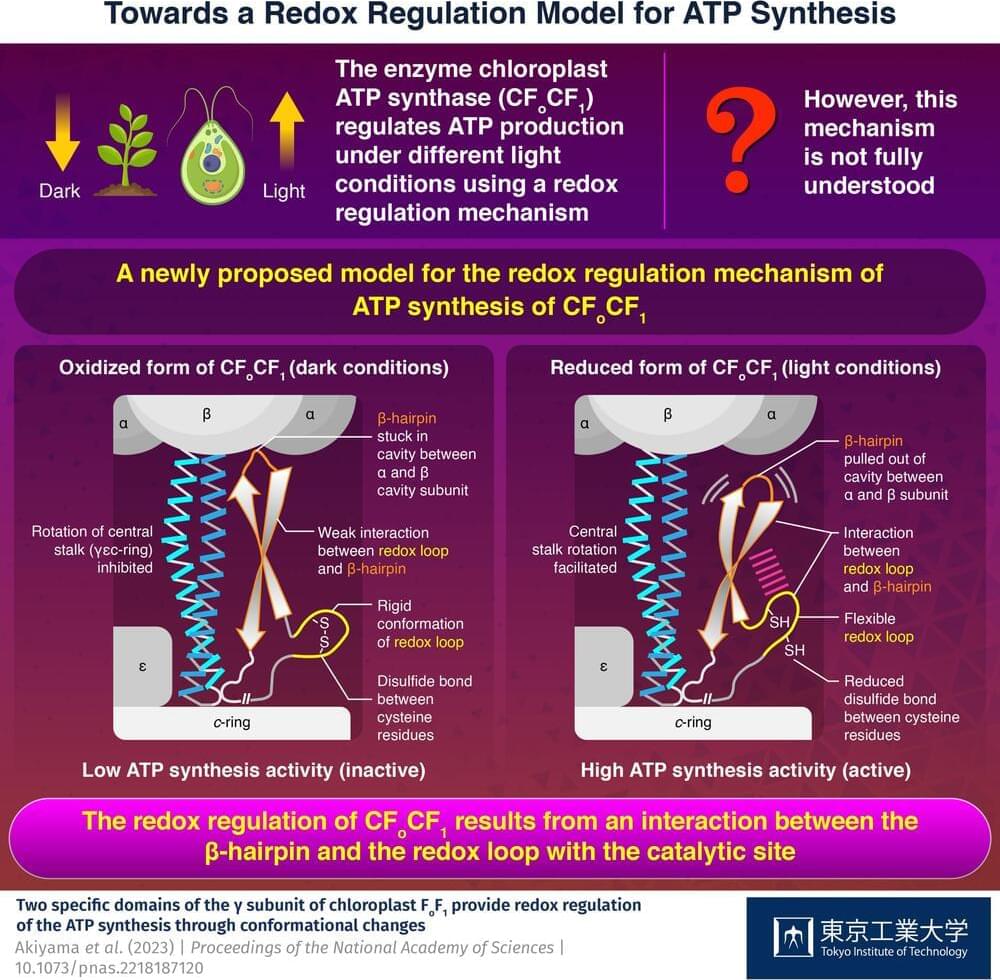
ATP, the compound essential for the functioning of photosynthetic organisms such as plants, algae, and cyanobacteria, is produced by an enzyme called “chloroplast ATP synthase” (CFoCF1). To control ATP production under varying light conditions, the enzyme uses a redox regulatory mechanism that modifies the ATP synthesis activity in response to changes in the redox state of cysteine (Cys) residues, which exist as dithiols under reducing (light) conditions, but forms a disulfide bond under oxidizing (dark) conditions. However, this mechanism has not yet been fully understood.
Now, in a study published in the Proceedings of the National Academy of Sciences, a team of researchers from Japan led by Prof. Toru Hisabori from Tokyo Institute of Technology (Tokyo Tech) has uncovered the role of the amino acid sequences present in CFoCF1, revealing how the enzyme regulates ATP production in photosynthetic organisms.
To understand how the conformation of the amino acids present in CFoCF1 contributes to the redox regulation mechanism, the researchers used the unicellular green alga, Chlamydomonas reinhardtii, to produce the enzyme. “By leveraging the powerful genetics of Chlamydomonas reinhardtii as a model organism for photosynthesis, we conducted a comprehensive biochemical analysis of the CFoCF1 molecule,” explains Prof. Hisabori.