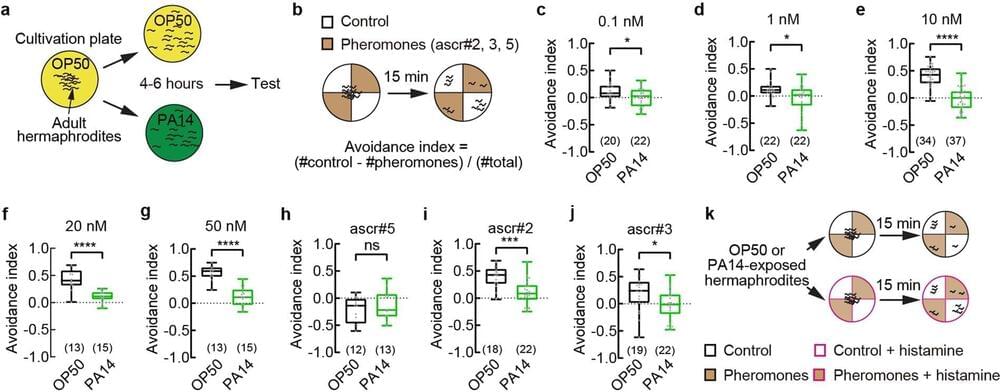
As COVID has demonstrated, when pathogens are moving through the population, we adjust, limiting interactions, even isolating, and generally changing the way we associate with one other. Humans are not alone. New research from Harvard scientists provides some insight into how pathogens change animal social behaviors.
“Extreme environmental conditions have a very strong influence on all animals,” said Yun Zhang, a professor in the Department of Organismic and Evolutionary Biology. But while this behavior has been seen in animals from simple fruit flies all the way up to primates, researchers have not understood what happens inside an individual animal’s brain that leads to infection-induced changes in social behavior.
In their new paper, published in Nature, Zhang and colleagues studied the small roundworm C. elegans, which exists in nature with two sexes: hermaphrodites that produce both eggs and sperm, and males. Under normal conditions, the hermaphrodites are loners, preferring to self-reproduce over mating with males. However, Zhang’s team found that the hermaphrodite worms infected by a pathogenic strain of the bacterium Pseudomonas aeruginosa became more interested in one another and increased their mating with males.