Commercial platforms for protein therapeutics are being built on academic research that has expanded the genetic code behind cell-based translation.

Antibiotic resistance is a major danger to public health that threatens to claim the lives of millions of people per year within the next few decades. Years of necessary administration and excessive application of antibiotics have selected for strains that are resistant to many of our currently available treatments. Due to the high costs and difficulty of developing new antibiotics, the emergence of resistant bacteria is outpacing the introduction of new drugs to fight them. To overcome this problem, many researchers are focusing on developing antibacterial therapeutic strategies that are “resistance-resistant”—regimens that slow or stall resistance development in the targeted pathogens. In this mini review, we outline major examples of novel resistance-resistant therapeutic strategies. We discuss the use of compounds that reduce mutagenesis and thereby decrease the likelihood of resistance emergence. Then, we examine the effectiveness of antibiotic cycling and evolutionary steering, in which a bacterial population is forced by one antibiotic toward susceptibility to another antibiotic. We also consider combination therapies that aim to sabotage defensive mechanisms and eliminate potentially resistant pathogens by combining two antibiotics or combining an antibiotic with other therapeutics, such as antibodies or phages. Finally, we highlight promising future directions in this field, including the potential of applying machine learning and personalized medicine to fight antibiotic resistance emergence and out-maneuver adaptive pathogens.
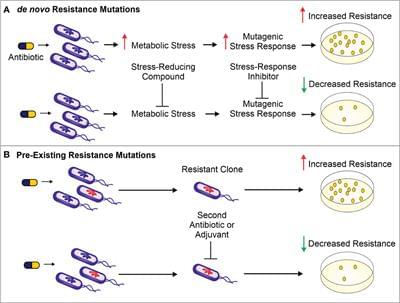
The use of antibiotics is central to the practice of modern medicine but is threatened by widespread antibiotic resistance (Centers for Disease Control and Prevention (U.S.), 2019). Antibiotics are a selective evolutionary pressure—they inhibit bacterial growth and viability, and antibiotic-treated bacteria are forced to either adapt and survive or succumb to treatment. The stress of antibiotic treatment can enhance bacterial mutagenesis leading to de novo resistance mutations (Figure 1A), promote the acquisition of horizontally transferred genetic elements that confer resistance, or trigger phenotypic responses that increase tolerance to drugs (Davies and Davies, 2010; Levin-Reisman et al., 2017; Bakkeren et al., 2019; Darby et al., 2022;). Additionally, antibiotic treatment can select for the proliferation of pre-existing mutants already in the population (Figure 1B).
Serious Science — http://serious-science.org.
Behavioral geneticist Robert Plomin on twin studies, genetic influence of parents on their children, and 1% of DNA that makes people different.

Ecologists have shown that the genetic material that species.
A species is a group of living organisms that share a set of common characteristics and are able to breed and produce fertile offspring. The concept of a species is important in biology as it is used to classify and organize the diversity of life. There are different ways to define a species, but the most widely accepted one is the biological species concept, which defines a species as a group of organisms that can interbreed and produce viable offspring in nature. This definition is widely used in evolutionary biology and ecology to identify and classify living organisms.

According to recent research published in the Journal of Experimental Medicine, equipping cancer-infecting viruses with tumor-inhibiting genetic cargo boosts the immune system and supports immunotherapy in reducing or totally eradicating aggressive tumours in mice. The findings pave the path for clinical studies combining oncolytic viruses with immunotherapy.
The study states that cancer-infecting viruses can boost immunity of the body and support immunotherapy.
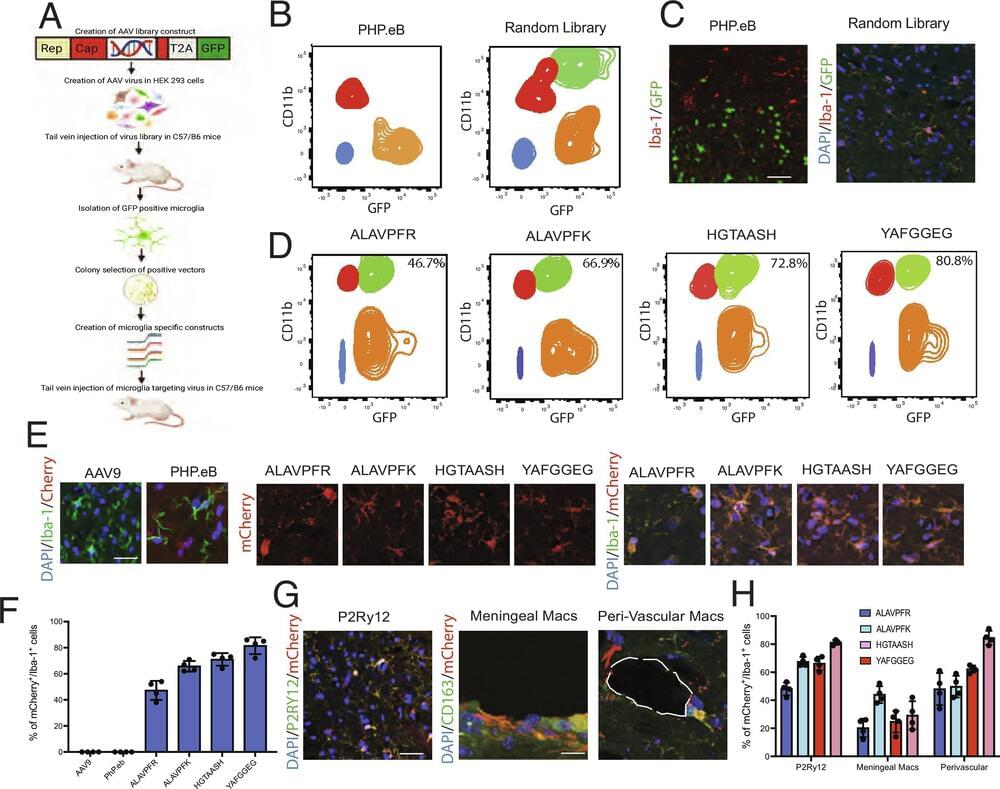
Cool paper that adds a useful tool to the gene therapist’s toolbox! Young et al. utilize an in vivo screening method to develop adeno-associated viruses (AAVs) which target microglia. They show that their AAVs transduce central nervous system microglia as well as tissue macrophages after intravenous injection. #biotechnology
Tissue macrophages, including microglia, are notoriously resistant to genetic manipulation. Here, we report the creation of Adeno-associated viruses (AAV) variants that efficiently and widely transduce microglia and tissue macrophages in vivo following intravenous delivery, with transgene expression of up to 80%. We use this technology to demonstrate manipulation of microglia gene expression and microglial ablation, thereby providing invaluable research tools for the study of these important cells.

This genetically engineered microorganism has the ability to break down a type of plastic known as polyethylene terephthalate (PET).
Various bacterial species have demonstrated an extraordinary ability to degrade plastics, which are synthetic polymers known for their long-lasting and non-biodegradable characteristics.
Research in this area continues to advance to create viable and sustainable solutions to combat the growing menace of plastic waste in terrestrial and marine environments.
Naked mole rats are rodents that are about the size of a mouse with a key difference, aside from having no fur — they’re extremely long-lived — reaching ages of around 40 years old. For comparison, lab mice live an average of about three and a half years. To explain their extensive lifespans, researchers have sought to pinpoint how naked mole rats evade the onset of age-related diseases like cancer. In doing so, they’ve identified a form of gelatinous substance called hyaluronan, which has anti-inflammatory and anticancer properties. Now, the question of whether the benefits of the naked mole rat’s abundant levels of this form of hyaluronan — called high molecular mass hyaluronic acid (HMM-HA) — can be exported to other species has recently drawn attention.
Published in Nature, Gorbunova and colleagues from the University of Rochester show that genetically modifying mice to harbor an enzyme that produces HMM-HA extends their lifespan. The researchers go on to show that increasing HMM-HA reduces the prevalence of cancer. Additionally, the nmrHAS2 gene improves the healthspan of mice by countering physiological dysfunction, as measured with a frailty score. These findings provide the first evidence that genes from long-lived species can be exported to other species, perhaps conferring benefits to humans one day.

The idea that genetic modification can improve humanity isn’t new, but it has taken some interesting turns within the scientific community over the past few years. One of the most notable comes from the mind of He Jiankui, a Chinese scientist whose gene editing of human babies led to infamy and a prison sentence. Now, He, known as JK to friends, thinks that gene-edited humans could be the future of our species.
Sign up for the most interesting tech & entertainment news out there.