Cell division is a crucial process for all life forms, from bacteria to blue whales, enabling growth, reproduction, and the continuation of species. Despite its universal nature, the methods of cell division vary significantly across organisms. A recent study by EMBL Heidelberg’s Dey group, along with their collaborators and published in Nature, investigates the evolution of cell division methods in organisms closely related to fungi and animals. For the first time, this research demonstrates the connection between an organism’s life cycle and its cell division techniques.
Despite last sharing a common ancestor over a billion years ago, animals and fungi are similar in many ways. Both belong to a broader group called ‘eukaryotes’ – organisms whose cells store their genetic material inside a closed compartment called the ‘nucleus’. The two differ, however, in how they carry out many physiological processes, including the most common type of cell division – mitosis.
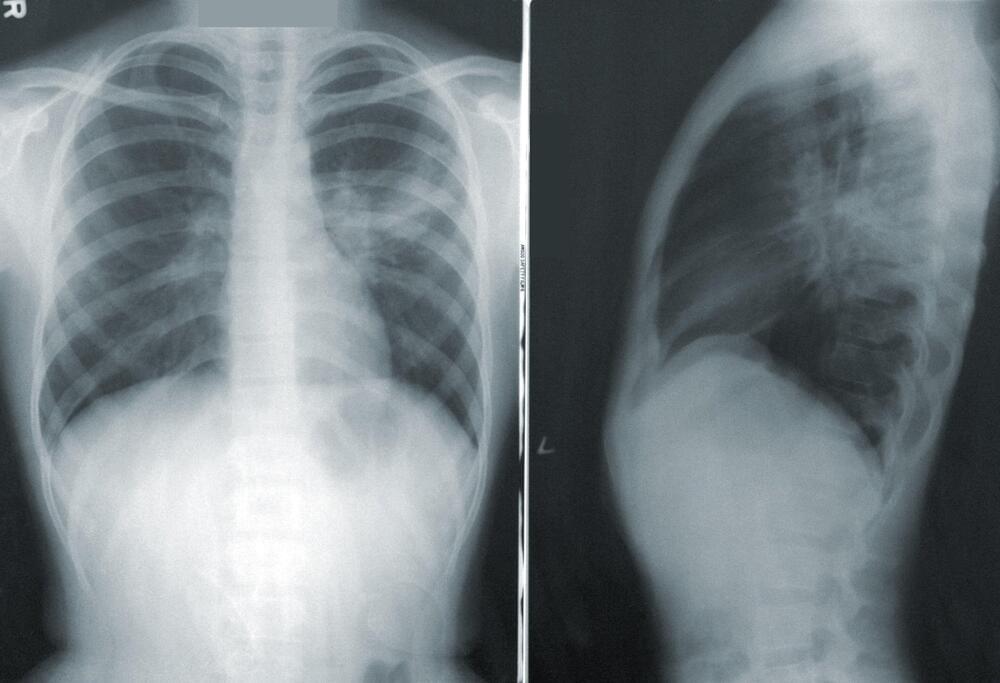
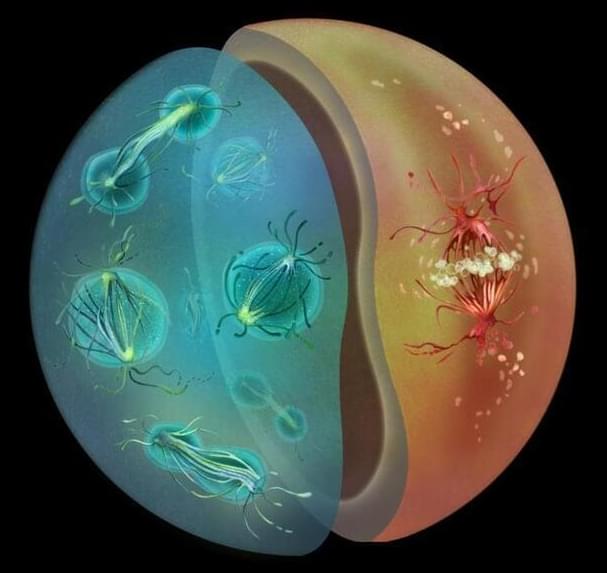
Most animal cells undergo ‘open’ mitosis, in which the nuclear envelope – the two-layered membrane separating the nucleus from the rest of the cell – breaks down when cell division begins. However, most fungi use a different form of cell division – called ‘closed’ mitosis – in which the nuclear envelope remains intact throughout the division process. However, very little is known about why or how these two distinct modes of cell division evolved and what factors determine which mode would be predominantly followed by a particular species.