The CRISPR gene-editing technique has revolutionised biology, but now an even more powerful system called bridge editing could let us completely reshape genomes.

The CRISPR gene-editing technique has revolutionised biology, but now an even more powerful system called bridge editing could let us completely reshape genomes.
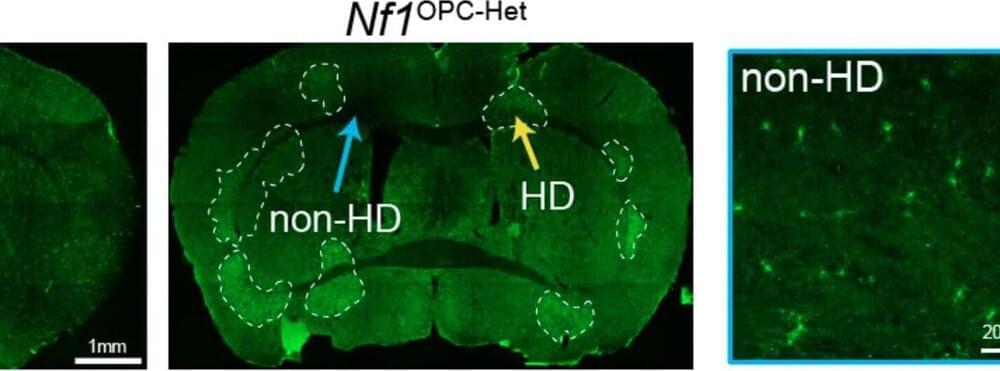
Neurogenetic disorders, such as neurofibromatosis type 1 (NF1), are diseases caused by a defect in one or more genes, which can sometimes result in cognitive and motor impairments. Better understanding the neural underpinning of these disorders and how they affect motor and cognitive abilities could contribute to the development of new treatment strategies.
Researchers at Stanford University and Washington University School of Medicine recently performed a study on mice aimed at investigating the impact of Nf1 gene mutations, which cause the NF1 neurogenetic disorder, on oligodendroglial plasticity, an adaptive brain process known to contribute to cognitive and motor functions.
Their findings, published in Nature Neuroscience, provide strong evidence that Nf1 mutations delay the development of oligodendroglia, a type of glial cells that support the functioning of the central nervous system, causing disruptions in motor learning.
An immunotherapy drug given before surgery instead of chemotherapy meant that over ten times more patients with a certain genetic profile were cancer-free after surgery, according to clinical trial results presented by researchers at UCL and UCLH.
The findings, presented at the American Society of Clinical Oncology (ASCO) Annual Meeting 2024, are interim results from the NEOPRISM-CRC phase II clinical trial assessing whether the immunotherapy drug pembrolizumab can improve outcomes for patients with stage two or stage three MMR deficient/MSI-High bowel cancer. The trial was a collaboration among UCL, UCLH, the Christie NHS Foundation Trust in Manchester, St. James’s University Hospital in Leeds, University Hospital Southampton and the University of Glasgow.
Bowel cancer is the fourth most common cancer in the UK, with around 42,900 cases a year. Though still predominantly a cancer that affects older people, cases among the under 50s have been increasing in recent decades.
The enzyme telomerase can prevent telomere attrition from happening by extending the length of telomeres. However, in most multicellular organisms, including humans, telomerase expression is switched off, except in germ cells, some types of stem cells, and certain white blood cells. While this might play a role in preventing cancer, as most cancerous cells must switch telomerase expression back on via mutations to enable runaway replication, numerous studies have shown that increasing telomerase through TERT delays aging and increases longevity of model organisms [1].
The small molecule that could
In the lab, this is usually done by introducing genetic vectors carrying a working copy of the gene that codes TERT. It’s this gene that is switched off in somatic cells. However, gene therapies are complex and expensive, and they are just entering the medical mainstream. What if we could do the same using a small molecule?

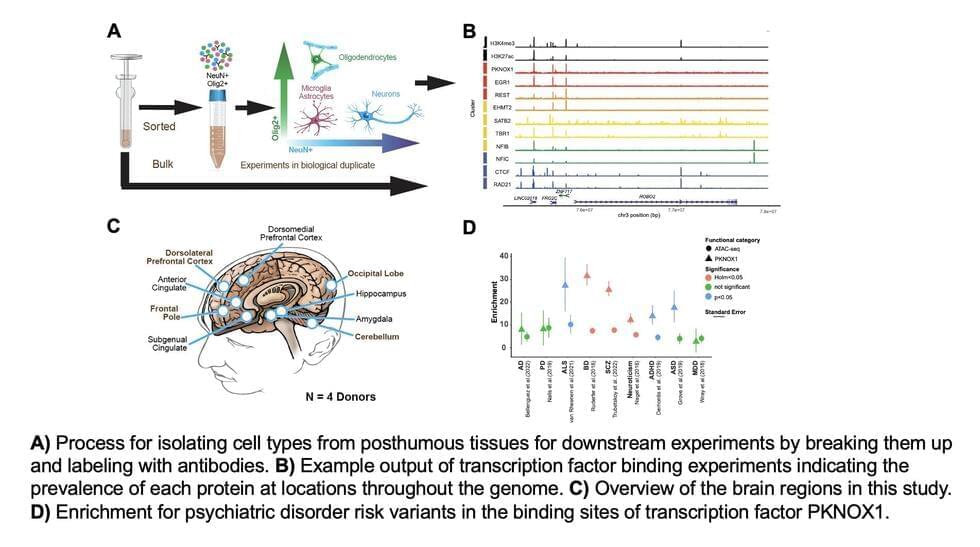
Transcription factors (TFs) are proteins that bind to specific DNA sequences, regulating the transcription of genetic information from DNA to messenger RNA (mRNA). These proteins play a pivotal role in the regulation of gene expression, which in turn impacts a wide range of biological processes and brain functions.

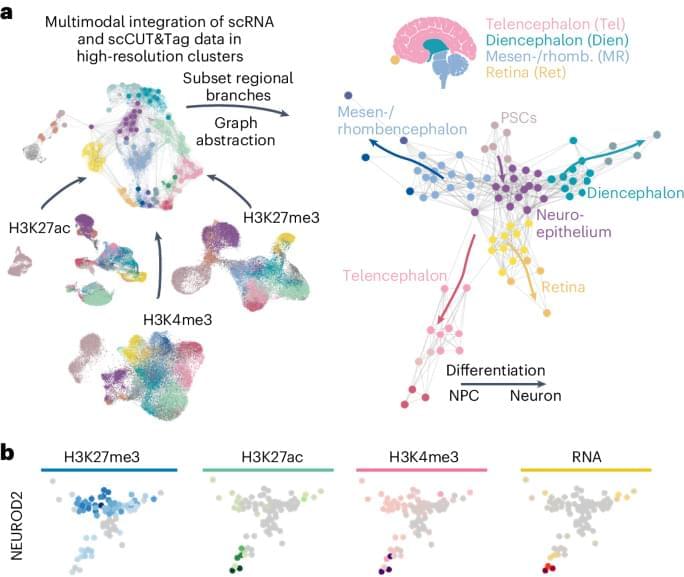
We present a developmental atlas that offers insight into sequential epigenetic changes underlying early human brain development modeled in organoids, which reconstructs the differentiation trajectories of all major CNS regions. It shows that epigenetic regulation via the installation of activating histone marks precedes activation of groups of neuronal genes.
Centenarians have become the fastest-growing demographic group in the world, with numbers approximately doubling every 10 years since the 1970s.
Many researchers have sought out the factors and contributors that determine a long and healthy life. The dissolution isn’t new either, with Plato and Aristotle writing about the ageing process over 2,300 years ago.
Understanding what is behind living a longer life involves unravelling the complex interplay of genetic predisposition and lifestyle factors and how they interact.
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhDDiscount Links: Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7x…