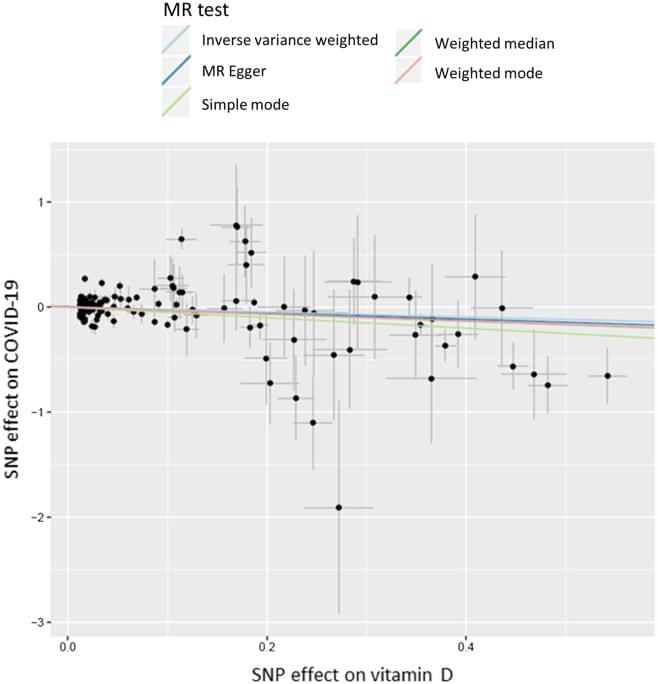
The discriminatory power of the UVB variable is somewhat limited in this study, because UVB radiation is low at this time of the year, particularly at the high northern latitude of UK—larger effects might be observed if variation in UVB is greater. We only used ambient UVB, and did not capture individual behavioural differences that would determine the actual level of vitamin D synthesis in the skin, such as duration and time of day spent outside, clothing, etc. It is important to note that time of year is the strongest predictor of vitD-UVB. To avoid bias control dates were assigned to follow the same distribution as case dates, which might have led to artificially diminished differences in vitD-UVB between cases and controls, however analysis relating to hospitalisation and death are not affected by this. We also conducted an analysis of the genetically-predicted vitamin D and a number of state-of-the-art MR analyses. However, the main limitation is the lack of power. Given the small number of COVID-19 patients and the relatively small percentage of variance (4.2%) explained by vitamin D-related genetic variants, this MR study was not adequately powered to detect small causal effect and negative results should be interpreted with caution. Additionally, MR studies only consider linear effects between 25-OHD levels and COVID-19 risk, which do not capture what happens at the extremes of vitamin D deficiency. Therefore, it cannot rule out the possibility that seriously ill patients (due to an underlying pathology) with extremely low vitamin D levels could be predisposed to COVID-19 infection and increased COVID-19 severity and mortality. Furthermore, 25-OHD levels are the used biomarker of vitamin D status in the study population, nevertheless, they correlate poorly with the active form of vitamin D (1,25-OH2D), which exerts the effects of vitamin D on a cellular level. Thus, this study cannot exclude effects of 1,25-OH2D on COVID-19 risk.
Another limitation of this cohort relates to the fact that not all participants have been tested for present (or past) COVID-19 infection; consequentially, taking participants who were not tested as controls could be a potential source of bias, given that misclassification of controls might be substantial due to the presence of asymptomatic infected individuals, further driving our findings to the null. This is evident from the 1:2 ratio between outpatient vs. inpatient cases. It should be acknowledged that the COVID-19 cases in UK biobank have a high rate of hospitalisation due to the very limited and targeted testing at this stage of the pandemic in the UK, so this study reflects mainly those with more severe COVID-19 and gives less information about true infection risk, or risk of milder disease. In addition, we excluded individuals with a negative COVID-19 testing result from the controls due to the risk of those being false negatives. Although there is a risk of introducing selection bias, we believe that the risk of introducing misclassification bias if we included them in the analysis could be higher22,23. Additionally, given the presence of asymptomatic infected individuals, taking participants who were not tested as controls could also be another potential source of bias. Our study assessed the effect of genetically predicted vitamin D levels on COVID-19 risk while taking into consideration of ambient UVB radiation during the pandemic. We show an indication of an inverse association between genetically predicted vitamin D levels and severe COVID-19. Findings from our study are consistent with a recent randomised controlled trial (RCT) that found protective effect of vitamin D supplementation among those hospitalised with COVID-1924. However, other clinical trials did not show an effect. For instance, a randomised trial of 240 patients showed that supplementation with a single very large dose of 200,000 IU of vitamin D3 that increased serum vitamin D levels (21–44 ng/ml) was nonetheless ineffective in decreasing the length of hospital stay or any other clinical outcomes among hospitalized patients with severe COVID-1925. It has been estimated that one SD change in standardized natural-log transformed 25-OHD levels corresponds to a change in 25-OHD levels of 29.2 nmol/l in vitamin D insufficient individuals (serum 25-OHD levels 50 nmol/l), which is comparable to the 21.2 nmol/l mean increase in 25-OHD levels conferred by taking daily 400 IU of cholecalciferol, the amount of vitamin D most often found in vitamin D supplements26. This estimation has clinical implication on the dose of vitamin D supplement for disease prevention. Given the lack of highly effective therapies against COVID-19, it is important to remain open-minded to emerging results from rigorously conducted studies of vitamin D.
In conclusion, we found no significant associations between COVID-19 risk and measured 25-OHD levels after adjusted for covariates, but this finding is limited by the fact that the vitamin D levels were measured on average 11 years before the pandemic. Ambient UVB was strongly and inversely associated with COVID-19 hospitalization and death. The main MR analysis did not show that genetically-predicted vitamin D levels were causally associated with COVID-19 risk, although MR sensitivity analyses indicated a potential causal effect. Overall, the effect of vitamin D levels on the risk or severity of COVID-19 remains controversial, further studies are needed to validate vitamin D supplementation as a means of protecting against worsened COVID-19.