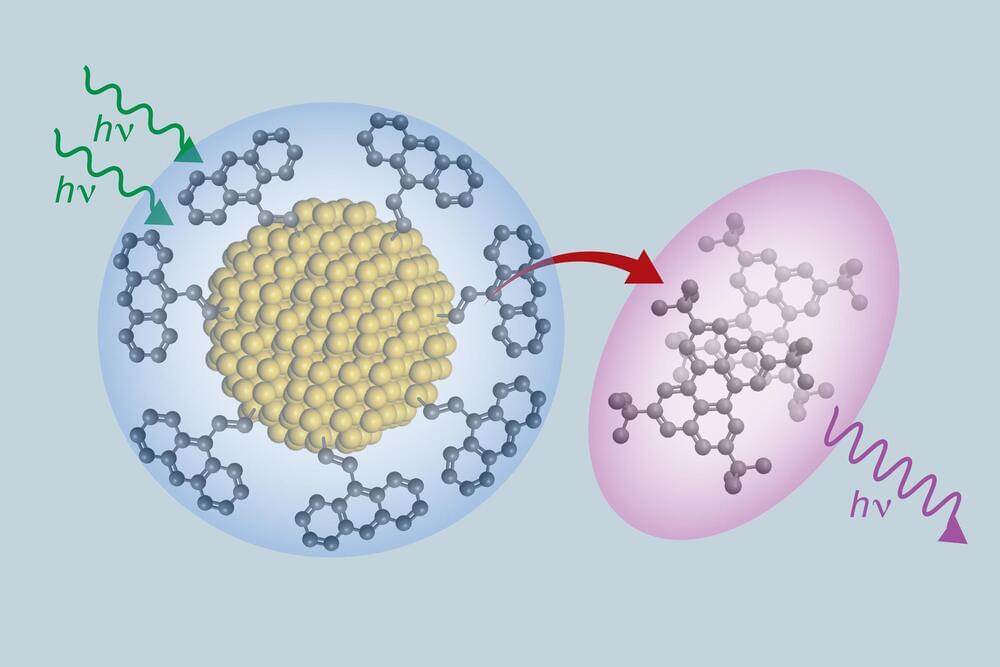
A group of scientists and engineers that includes researchers from The University of Texas at Austin have created a new class of materials that can absorb low energy light and transform it into higher energy light. The new material is composed of ultra-small silicon nanoparticles and organic molecules closely related to ones utilized in OLED TVs. This new composite efficiently moves electrons between its organic and inorganic components, with applications for more efficient solar panels, more accurate medical imaging and better night vision goggles.
The material is described in a new paper in Nature Chemistry.
“This process gives us a whole new way of designing materials,” said Sean Roberts, an associate professor of chemistry at UT Austin. “It allows us to take two extremely different substances, silicon and organic molecules, and bond them strongly enough to create not just a mixture, but an entirely new hybrid material with properties that are completely distinct from each of the two components.”