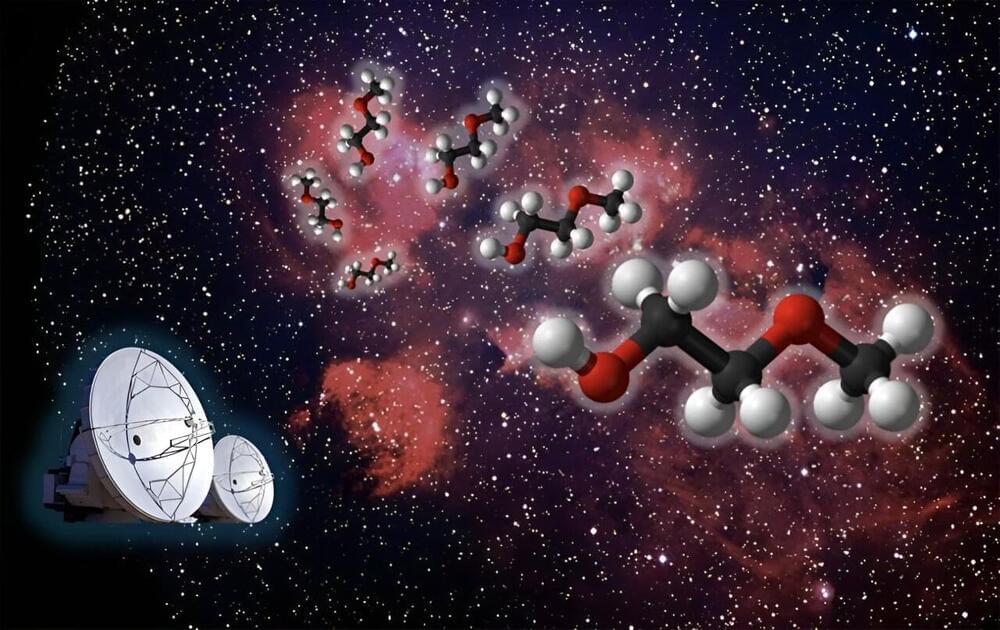
New research from the group of MIT Professor Brett McGuire has revealed the presence of a previously unknown molecule in space. The team’s open-access paper, “Rotational Spectrum and First Interstellar Detection of 2-Methoxyethanol Using ALMA Observations of NGC 6334I,” was published in the April 12 issue of The Astrophysical Journal Letters.
Zachary T.P. Fried, a graduate student in the McGuire group and the lead author of the publication, worked to assemble a puzzle comprised of pieces collected from across the globe, extending beyond MIT to France, Florida, Virginia, and Copenhagen, to achieve this exciting discovery.
“Our group tries to understand what molecules are present in regions of space where stars and solar systems will eventually take shape,” explains Fried. “This allows us to piece together how chemistry evolves alongside the process of star and planet formation. We do this by looking at the rotational spectra of molecules, the unique patterns of light they give off as they tumble end-over-end in space.