The association between this mass concentration and the idea that atoms are empty stems from a flawed view that mass is the property of matter that fills a space. However, this concept does not hold up to close inspection, not even in our human-scale world. When we pile objects on top of each other, what keeps them separated is not their masses but the electric repulsion between the outmost electrons at their touching molecules. (The electrons cannot collapse under pressure due to the Heisenberg uncertainty and Pauli exclusion principles.) Therefore, the electron’s electric charge ultimately fills the space.
Anyone taking Chemistry 101 is likely to be faced with diagrams of electrons orbiting in shells.
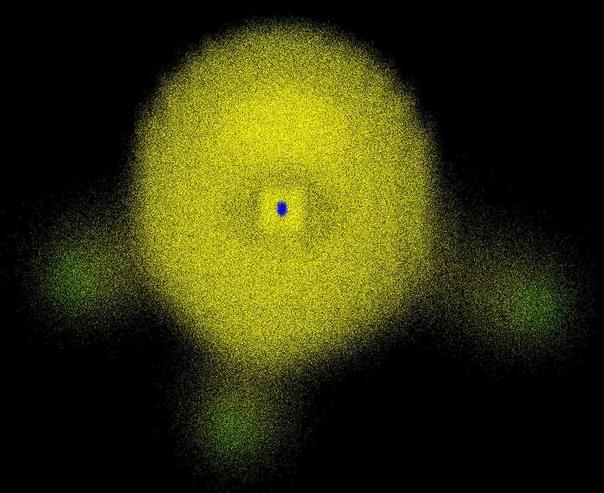
In atoms and molecules, electrons are everywhere! Look how the yellow cloud permeates the entire molecular volume in Figure 1. Thus, when we see that atoms and molecules are packed with electrons, the only reasonable conclusion is that they are filled with matter, not the opposite.