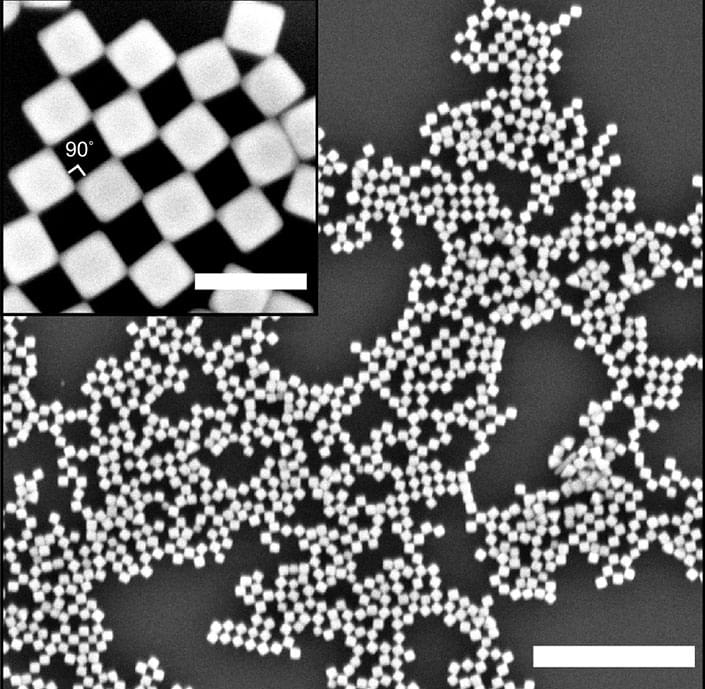
Researchers have engineered nanosized cubes that spontaneously form a two-dimensional checkerboard pattern when dropped on the surface of water. The work, published in Nature Communications (“Self-assembly of nanocrystal checkerboard patterns via non-specific interactions”), presents a simple approach to create complex nanostructures through a technique called self-assembly.
“It’s a cool way to get materials to build themselves,” said study co-senior author Andrea Tao, a professor in the Aiiso Yufeng Li Family Department of Chemical and Nano Engineering at the University of California San Diego. “You don’t have to go into a nanofabrication lab and do all these complex and precise manipulations.”
Each nanocube is composed of a silver crystal with a mixture of hydrophobic (oily) and hydrophilic (water-loving) molecules attached to the surface. When a suspension of these nanocubes is introduced to a water surface, they arrange themselves such that they touch at their corner edges. This arrangement creates an alternating pattern of solid cubes and empty spaces, resulting in a checkerboard pattern.