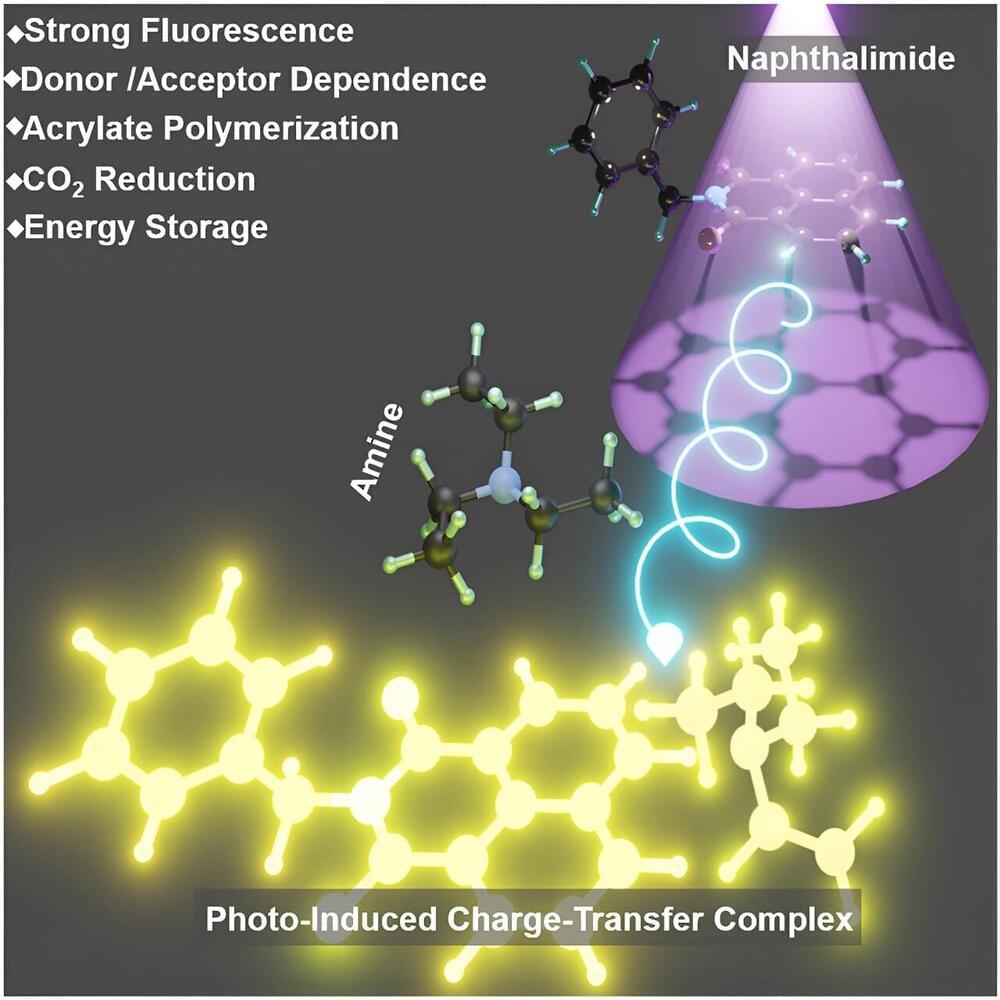
A research team led by Prof. Zhang Guoqing from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) has discovered a highly reactive photo-induced charge-transfer complex (PCTC) between amine and imide. Their findings are published in the journal Chem.
Charge transfer between molecules, a critical process in both natural and synthetic systems, plays a fundamental role in photosynthesis, respiration, and various organic synthesis and energy conversion applications.
Despite extensive research, creating stable, light-responsive charge-transfer complexes in artificial systems remains challenging. The discovery of PCTCs addresses this challenge, offering new insights into complex photochemical processes.