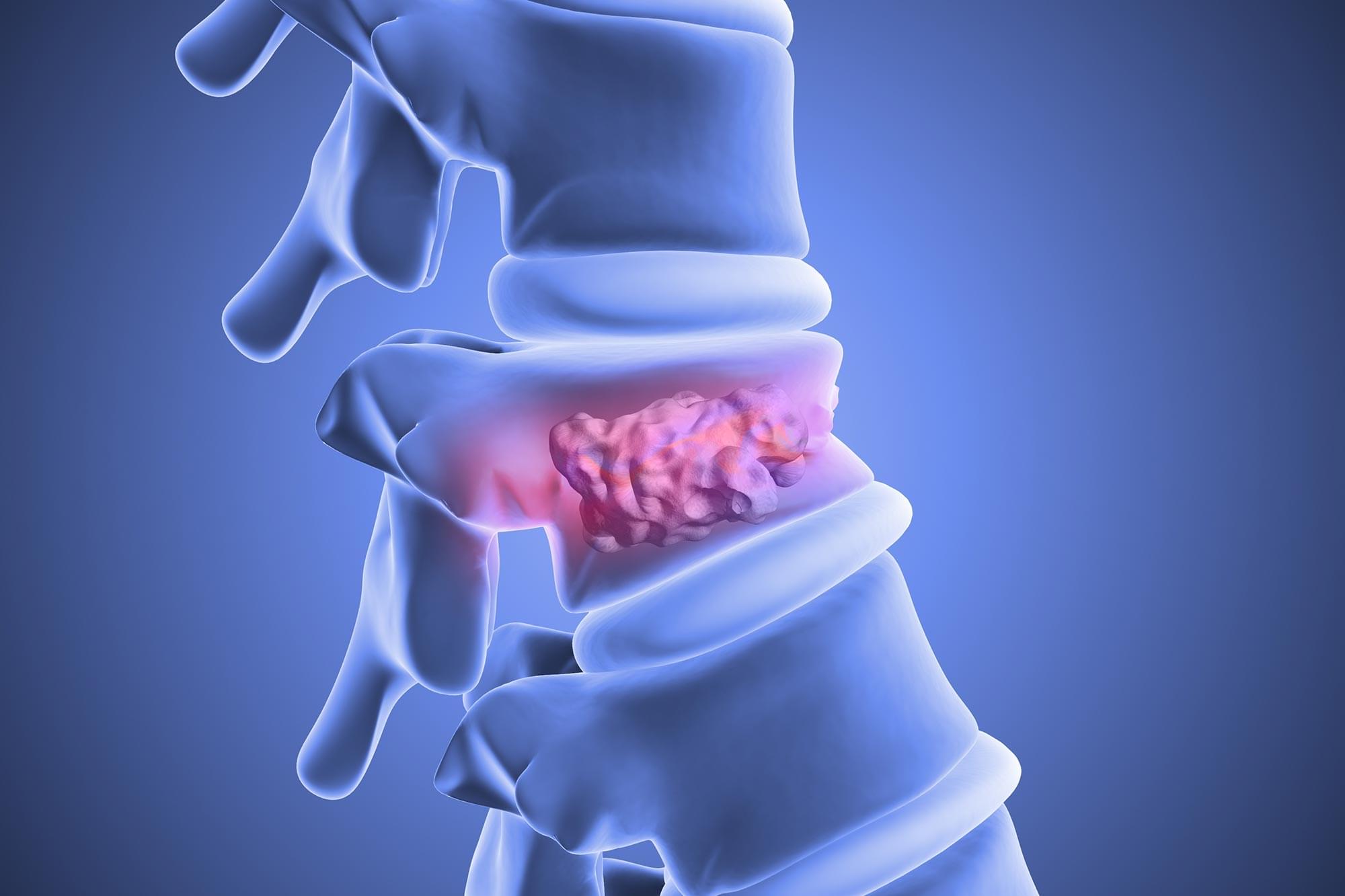
Fat stem cells may hold the key to repairing fragile spines and reversing bone loss.

Specific immune cells in the brain may play a crucial role in preventing the onset of Alzheimer’s disease, according to a new study – a discovery that could lead to new therapies that try to coax cells into this protective state.
Earlier studies have shown that immune cells in the brain called microglia can effectively tackle the symptoms of Alzheimer’s, but also make them worse through inflammation.
Here, an international team of scientists took a detailed look at how microglia switch between those two helpful and harmful modes.
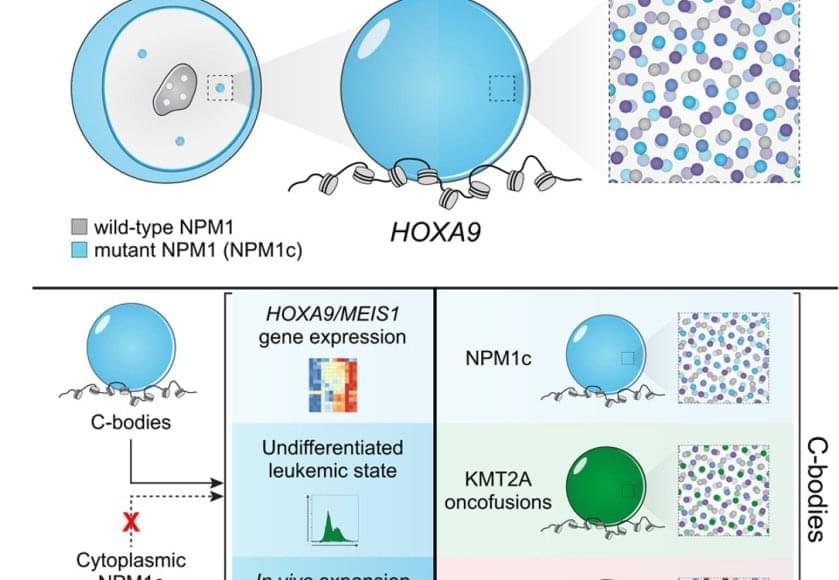
Leukemia starts when mutations in blood-forming cells disrupt the balance between growth and differentiation. Patients with entirely different genetic changes show strikingly similar patterns of gene activity and can respond to the same drugs. What invisible thread could make so many mutations behave the same way?
The authors looked into high-resolution microscope and saw something no one expected: leukemia cell nuclei shimmered with a dozen bright dots – tiny beacons missing from healthy cells.
Those dots weren’t random. They contained large amounts of mutant leukemia proteins and drew in many normal cell proteins to coordinate activation of the leukemia program. The dots were new nuclear compartments formed by phase separation, the same physical principle that describes why oil droplets form in water. The team named this new compartment, “coordinating bodies,” or C-bodies.
Inside the nucleus, these C-bodies act like miniature control rooms, pulling together the molecules that keep leukemia genes switched on. Like drops of oil collecting on the surface of soup, they appear when the cell’s molecular ingredients reach just the right balance.
Even more surprising, cells carrying entirely different leukemia mutations formed droplets with the same behavior. Although their chemistry differs, the resulting nuclear condensates perform the same function, using the same physical playbook.
A new quantitative assay confirmed it. These droplets are biophysically indistinguishable – like soups made from different ingredients that still simmer into the same consistency. No matter which mutation started the process, each leukemia formed the same kind of C-body.
The team confirmed the finding across human cell lines, mouse models and patient samples. When they tweaked the proteins so they could no longer form these droplets – or dissolved them with drugs, the leukemia cells stopped dividing and began to mature into healthy blood cells.


Statins have transformed heart health, saving millions of lives by lowering cholesterol and reducing the risk of heart attacks and strokes. But for many patients, these drugs come with a troubling downside: muscle pain, weakness and, in rare cases, severe muscle breakdown that can lead to kidney failure.
University of British Columbia researchers and their collaborators at the University of Wisconsin-Madison have now pinpointed the cause. Their findings, published last week in Nature Communications, could pave the way for a new generation of statins without these side effects.
A recent study points to a key bone-strengthening mechanism at work in the body, which could be targeted to treat the bone-weakening disease, osteoporosis.
Led by researchers from the University of Leipzig in Germany and Shandong University in China, the study identified the cell receptor GPR133 (also known as ADGRD1) as being crucial to bone density, via bone-building cells called osteoblasts.
Variations in the GPR133 gene had previously been linked to bone density, leading scientists to turn their attention to the protein it encoded.

Introduction: Imaging surveillance of contrast-enhancing lesions after the treatment of malignant brain tumors with radiation is plagued by an inability to reliably distinguish between tumor recurrence and treatment effects. Magnetic resonance perfusion-weighted imaging (PWI)—among other advanced brain tumor imaging modalities—is a useful adjunctive tool for distinguishing between these two entities but can be clinically unreliable, leading to the need for tissue sampling to confirm diagnosis. This may be partially because clinical PWI interpretation is non-standardized and no grading criteria are used for assessment, leading to interpretation discrepancies. This variance in the interpretation of PWI and its subsequent effect on the predictive value has not been studied. Our objective is to propose structured perfusion scoring criteria and determine their effect on the clinical value of PWI. Methods: Patients treated at a single institution between 2012 and 2022 who had prior irradiated malignant brain tumors and subsequent progression of contrast-enhancing lesions determined by PWI were retrospectively studied from CTORE (CNS Tumor Outcomes Registry at Emory). PWI was given two separate qualitative scores (high, intermediate, or low perfusion). The first (control) was assigned by a neuroradiologist in the radiology report in the course of interpretation with no additional instruction. The second (experimental) was assigned by a neuroradiologist with additional experience in brain tumor interpretation using a novel perfusion scoring rubric. The perfusion assessments were divided into three categories, each directly corresponding to the pathology-reported classification of residual tumor content. The interpretation accuracy in predicting the true tumor percentage, our primary outcome, was assessed through Chi-squared analysis, and inter-rater reliability was assessed using Cohen’s Kappa. Results: Our 55-patient cohort had a mean age of 53.5 ± 12.2 years. The percentage agreement between the two scores was 57.4% (κ: 0.271). Upon conducting the Chi-squared analysis, we found an association with the experimental group reads (p-value: 0.014) but no association with the control group reads (p-value: 0.734) in predicting tumor recurrence versus treatment effects. Conclusions: With our study, we showed that having an objective perfusion scoring rubric aids in improved PWI interpretation. Although PWI is a powerful tool for CNS lesion diagnosis, methodological radiology evaluation greatly improves the accurate assessment and characterization of tumor recurrence versus treatment effects by all neuroradiologists. Further work should focus on standardizing and validating scoring rubrics for PWI evaluation in tumor patients to improve diagnostic accuracy.
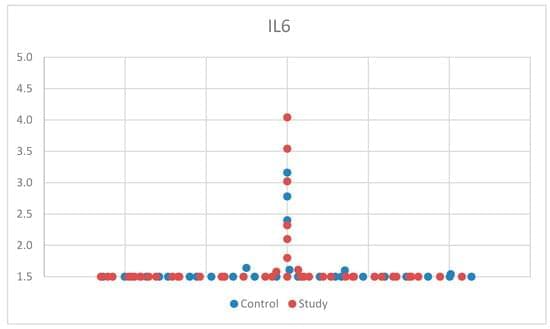
Background: Autism spectrum disorder (ASD) has seen a rise in prevalence, and the immune system’s role in brain development is increasingly recognized. This study investigates the relationship between immune dysregulation and ASD by examining serum concentrations of interleukin 6 (IL-6), interleukin 8 (CXCL8), and tumor necrosis factor alpha (TNF-alpha) in children. Methods: Serum samples from 45 children with ASD and 30 controls, aged 2 to 12 years, were analyzed using electrochemiluminescence, chemiluminescent microparticle immunoassay, and chemiluminescent immunoassay. ASD symptoms were assessed using the Autism Spectrum Rating Scale (ASRS) and Social Communication Questionnaire (SCQ). Results: No significant correlation was observed between CXCL8 levels and ASD. IL-6 levels showed a trend toward elevation in boys with ASD.