Palliative care, a type of healthcare for patients with life-threatening diseases, focuses on improving quality of life and managing pain. Unlike hea | Cancer




Scientists at UCSF have uncovered how certain immune cells in the brain, called microglia, can effectively digest toxic amyloid beta plaques that cause Alzheimer’s. They identified a key receptor, ADGRG1, that enables this protective action. When microglia lack this receptor, plaque builds up quickly, causing memory loss and brain damage. But when the receptor is present, it seems to help keep Alzheimer's symptoms mild. Since ADGRG1 belongs to a drug-friendly family of receptors, this opens the door to future therapies that could enhance brain immunity and protect against Alzheimer’s in more people.
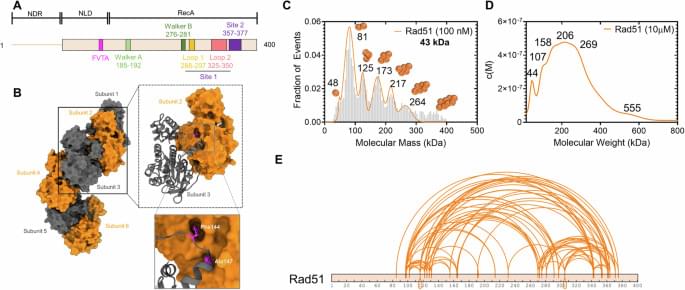
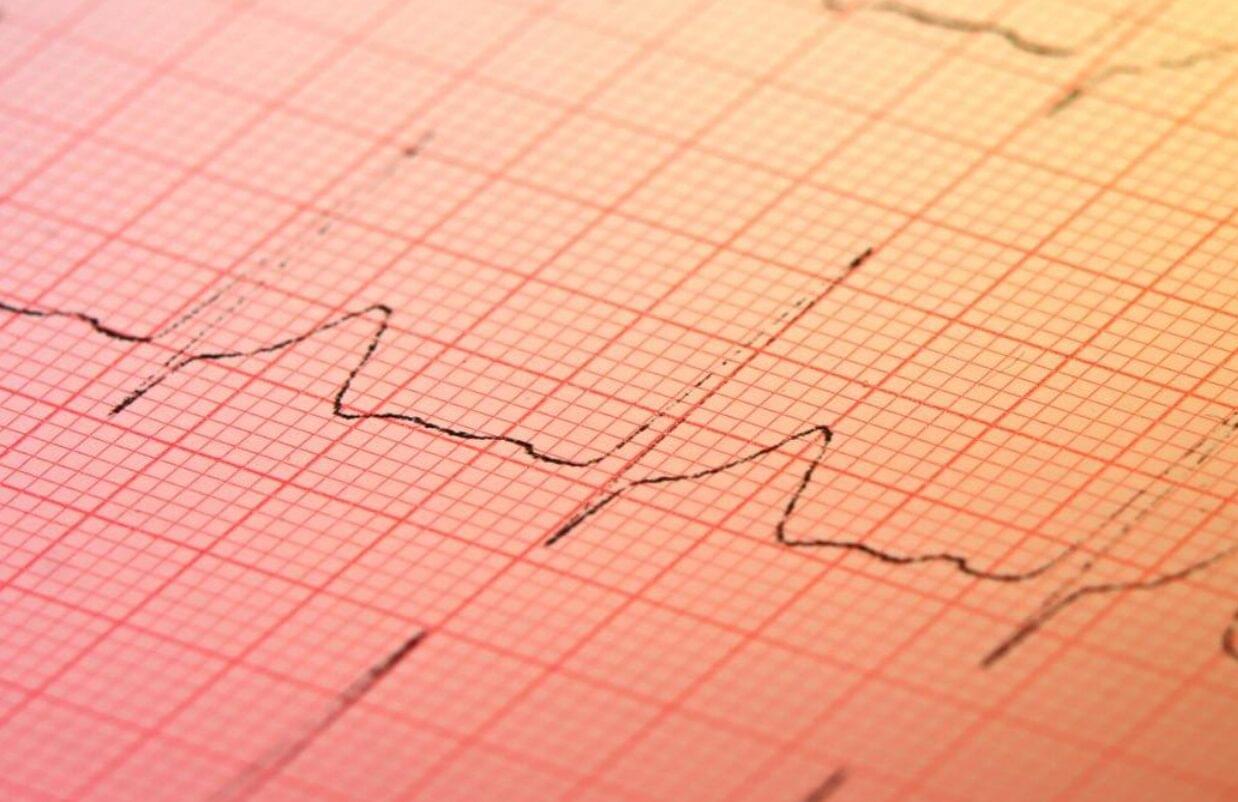
With the help of artificial intelligence (AI), an inexpensive test found in many doctors’ offices may soon be used to screen for hidden heart disease.
Structural heart disease, including valve disease, congenital heart disease, and other issues that impair heart function, affects millions of people worldwide. Yet in the absence of a routine, affordable screening test, many structural heart problems go undetected until significant function has been lost.
“We have colonoscopies, we have mammograms, but we have no equivalents for most forms of heart disease,” says Pierre Elias, assistant professor of medicine and biomedical informatics at Columbia University Vagelos College of Physicians and Surgeons and medical director for artificial intelligence at NewYork-Presbyterian.
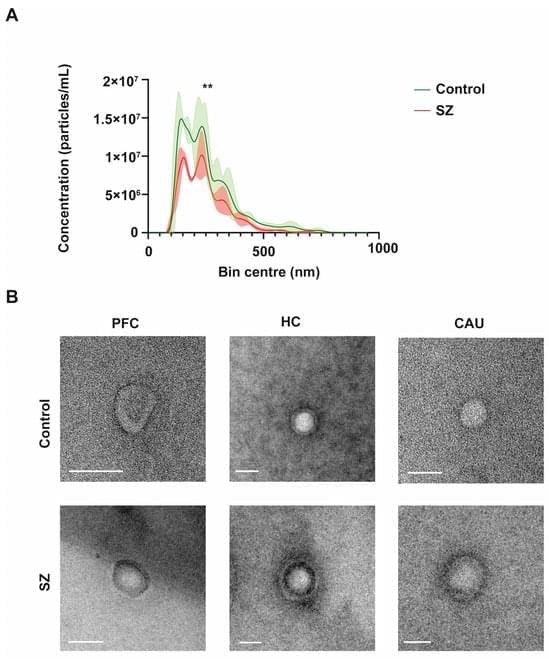
Extracellular vesicles (EVs) are tiny membranous structures that mediate intercellular communication. The role(s) of these vesicles have been widely investigated in the context of neurological diseases; however, their potential implications in the neuropathology subjacent to human psychiatric disorders remain mostly unknown. Here, by using next-generation discovery-driven proteomics, we investigate the potential role(s) of brain EVs (bEVs) in schizophrenia (SZ) by analyzing these vesicles from the three post-mortem anatomical brain regions: the prefrontal cortex (PFC), hippocampus (HC), and caudate (CAU). The results obtained indicate that bEVs from SZ-affected brains contain region-specific proteins that are associated with abnormal GABAergic and glutamatergic transmission.
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links/Affiliates:
Blood testing (where I get the majority of my labs): https://www.ultalabtests.com/partners/michaellustgarten.
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
Clearly Filtered Water Filter: https://get.aspr.app/SHoPY
Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1
Use Code: CONQUERAGING
NAD+ Quantification: https://www.jinfiniti.com/intracellular-nad-test/

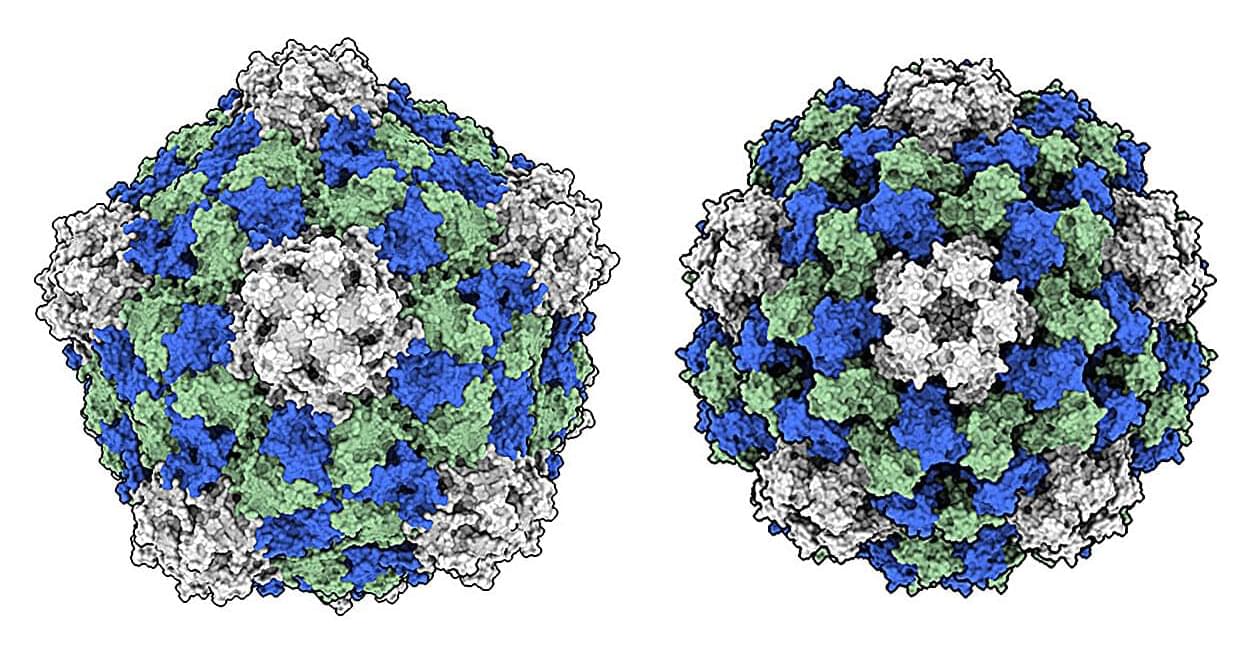
A virus that typically infects black-eyed peas is showing great promise as a low-cost, potent cancer immunotherapy—and researchers are uncovering why.
In a study published in Cell Biomaterials, a team led by chemical and nano engineers at the University of California San Diego took a closer look at how the cowpea mosaic virus (CPMV), unlike other plant viruses, is uniquely effective at activating the body’s immune system to recognize and attack cancer cells.
The study is titled “Comparative analyses for plant virus-based cancer immunotherapy drug development.”