Learn how Deep Science Ventures’ zero-data approach tackles biotech’s early funding challenges to speed innovation and reduce risk.


If it has seemed like more people you know are developing diabetes, you are right. The diabetes epidemic is not called an epidemic for nothing: According to the American Diabetes Association, over 10% of the U.S. population—approximately 38.4 million people—had diabetes in 2021, and 1.2 million more people get diagnosed each year.
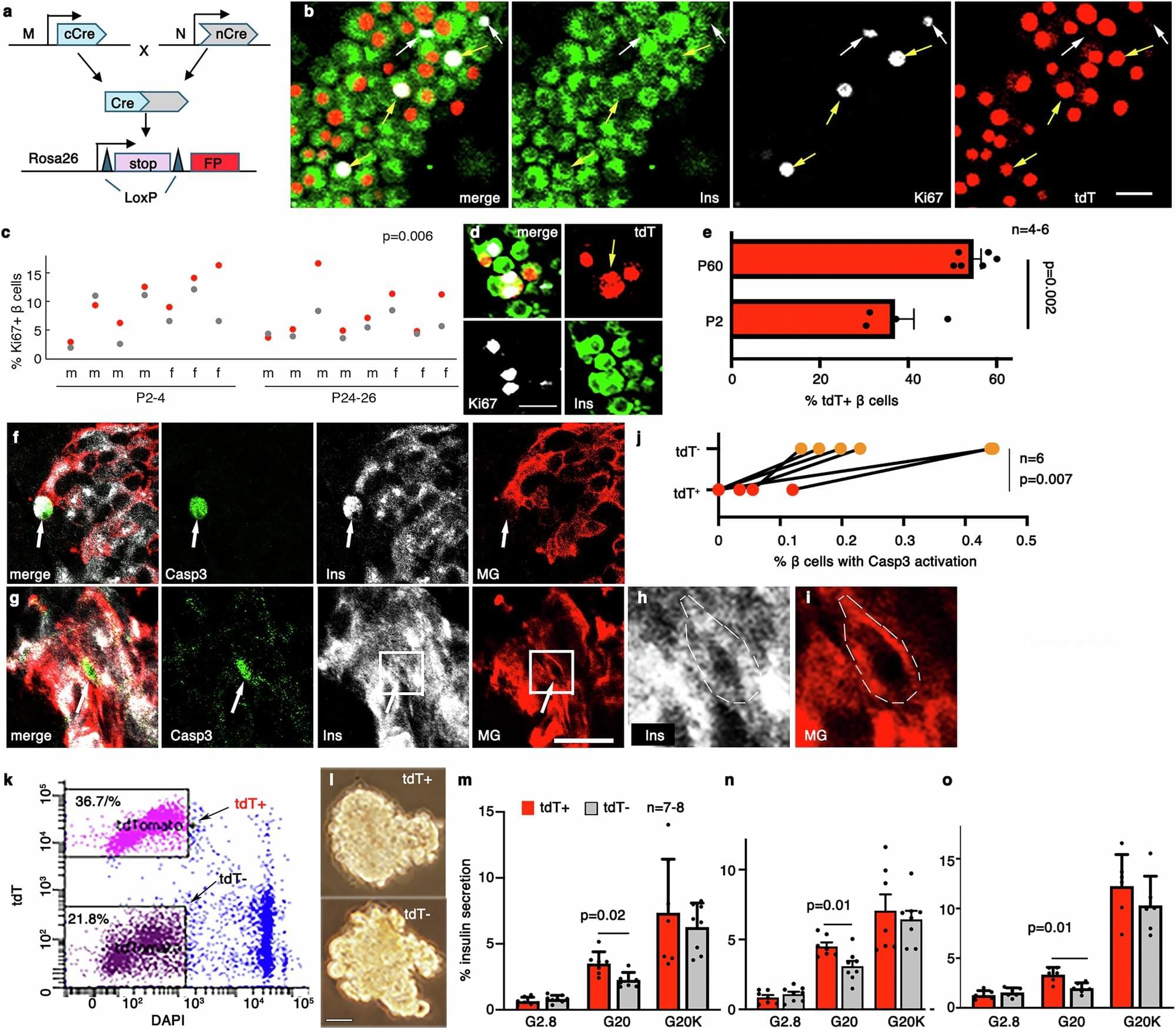
Type 2 diabetes occurs when your body develops a resistance to insulin, the hormone that helps regulate glucose levels in your blood. Insulin is secreted by pancreatic cells called β-cells, and in T2D, they ramp up insulin production to try to regulate blood glucose levels, but even that is insufficient and the β-cells eventually become exhausted over time. Thanks to their importance, the functional β-cell mass, or the total number of β-cells and their function, determines a person’s risk of diabetes.
Β-cells are not homogeneous, even within a single individual, and consist of different “subtypes,” each with their own secretory function, viability, and ability to divide. In other words, each β-cell subtype has a different level of fitness, and the higher, the better. When diabetes develops, the proportions of some β-cell subtypes are changed. But a key question remains: Are the proportion and fitness of different β-cell subtypes altered by diabetes or are the changes responsible for the disease?
Is Chief Executive Officer and Chairman of the Board of BioStem Technologies (https://biostemtechnologies.com/), a leading innovator focused on harnessing the natural properties of perinatal tissue in the development, manufacture, and commercialization of allografts for regenerative therapies.
Jason brings a wealth of experience in strategic operations planning and technical projects management from his rigorous technical background. His diverse expertise includes continuous process improvement, training and development programs, regulatory compliance and best practices implementation, and advanced problem solving.
Jason began his career as a technical engineer working for Adecco at SC Johnson in 2009, where he developed comprehensive maintenance plans to support manufacturing processes at scale. He then transitioned to manufacturing and quality engineering for major organizations, including ATI Ladish Forging, Nemak, and HUSCO International, where he spearheaded process design and implementation, solved complex supply-chain and manufacturing problems, and improved product sourcing and purchasing.
Jason’s philanthropic work with the Juvenile Diabetes Research Foundation sparked an interest in biotech, leading him to co-found Biostem Technologies in 2014. As CEO he has leveraged his expertise to optimize tissue sourcing, strategically build out a 6,000 square foot tissue processing facility that is fully compliant with FDA 210,211, 1,271, and AATB standards, and put together an expert team of professionals to support the company’s continued growth.
Jason holds a B.S. in Mechanical Engineering Technology and a minor in Mathematics from the Milwaukee School of Engineering and is Six Sigma Black Belt certified. He also serves as a Processing and Distribution Council Member for the American Association of Tissue Banks (AATB), as well as serves as a member of the Government Affairs committee for BioFlorida.
#JasonMatuszewski #BioStemTechnologies #PerinatalTissue #RegenerativeTherapies #ChronicWounds #DiabeticFootUlcers #VenousUlcers #PressureUlcers #AmnioticTissue #TissueAllografts #ExtracellularMatrix #ECM #GrowthFactors #Cytokines #Collagen #ProgressPotentialAndPossibilities #IraPastor #Podcast #Podcaster #ViralPodcast #STEM #Innovation #Technology #Science #Research
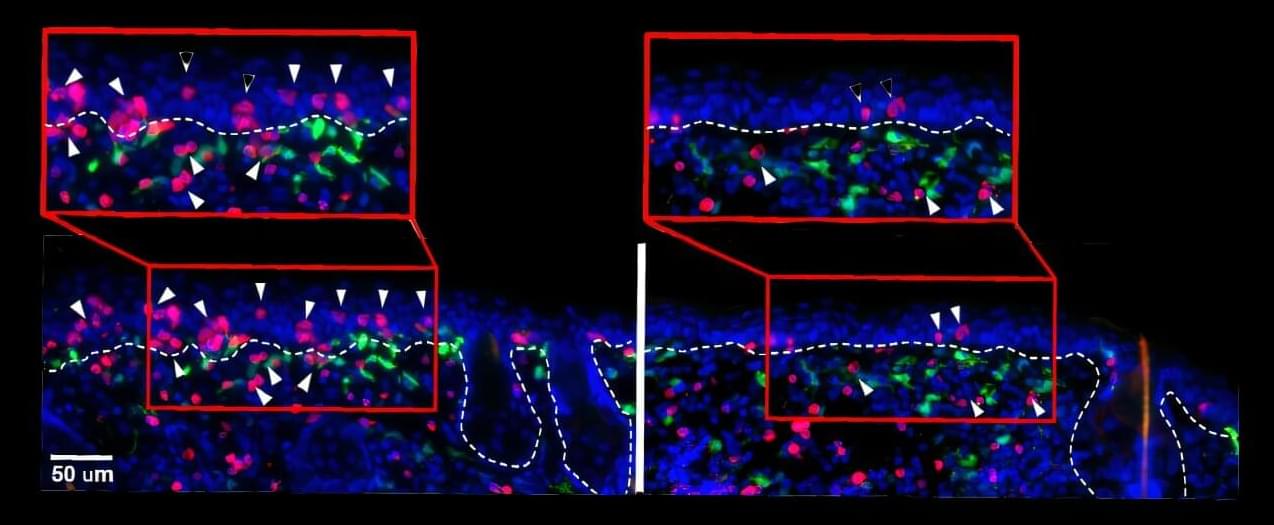
Weill Cornell Medicine researchers have discovered that PD-1—a molecule best known for putting the brakes on immune cells—also plays a critical role in helping T cells become long-term immune defenders in the skin. Early during infection, PD-1 acts like a steering wheel, guiding T cells to become protective resident memory T cells (TRM) that stay in place. These cells remember invading germs or cancer and quickly mount a response if that enemy reappears.
The preclinical findings, published July 29 in Nature Immunology, may impact how clinicians approach cancer treatments called immune checkpoint inhibitors. These drugs bind to PD-1 on the surface of T cells, releasing the brakes and unleashing the immune system to attack cancer cells.
Though immune checkpoint inhibitors are successful in treating melanoma, an aggressive skin cancer, about 40% of patients develop inflammatory rashes and itching in the skin or reactions in other epithelial tissues that cover internal and external surfaces of the body.
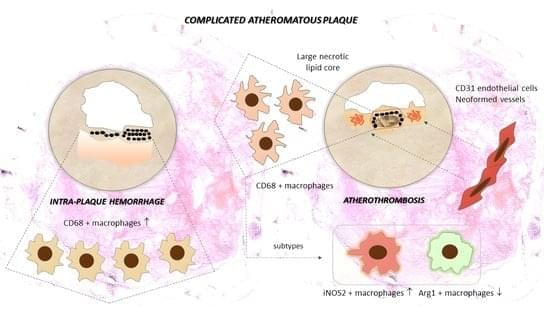
Background: Atherosclerosis is a progressive disease that results from endothelial dysfunction, inflammatory arterial wall disorder and the formation of the atheromatous plaque. This results in carotid artery stenosis and is responsible for atherothrombotic stroke and ischemic injury. Low-grade plaque inflammation determines biological stability and lesion progression. Methods: Sixty-seven cases with active perilesional inflammatory cell infiltrate were selected from a larger cohort of patients undergoing carotid endarterectomy. CD68+, iNOS2+ and Arg1+ macrophages and CD31+ endothelial cells were quantified around the atheroma lipid core using digital morphometry, and expression levels were correlated with determinants of instability: ulceration, thrombosis, plaque hemorrhage, calcification patterns and neovessel formation.

Ivermectin is typically used to treat neglected tropical diseases such as onchocerciasis (river blindness) and lymphatic filariasis (elephantiasis). However, studies have shown that it can also reduce malaria by killing mosquitoes that bite people who have taken the drug. As resistance to insecticides increases, ivermectin may offer a new and effective way to reduce transmission, especially in areas where standard methods are no longer reliable.
The BOHEMIA project (Broad One Health Endectocide-based Malaria Intervention in Africa), funded by Unitaid, tested this idea through two large-scale Mass Drug Administration (MDA) trials in regions with high malaria burden: Kwale County in Kenya and Mopeia district in Mozambique. Researchers evaluated whether giving a single monthly dose of ivermectin (400 mcg/kg) over three months at the start of the rainy season could lower malaria transmission. In Kenya, the program focused on children aged 5 to 15, while in Mozambique it targeted children under the age of five.
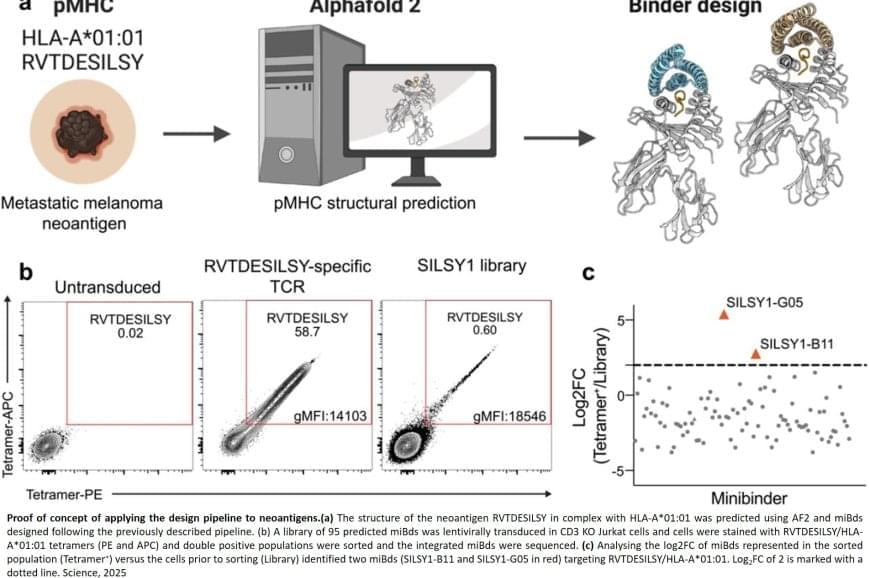
Normally, T cells naturally identify cancer cells by recognizing specific protein fragments, known as peptides, presented on the cell surface by molecules called pMHCs. It is a slow and challenging process to utilize this knowledge for therapy, often because the variation in the body’s own T-cell receptors makes it challenging to create a personalized treatment.
In the study, the researchers tested the strength of the AI platform on a well-known cancer target, NY-ESO-1, which is found in a wide range of cancers. The team succeeded in designing a minibinder that bound tightly to the NY-ESO-1 pMHC molecules. When the designed protein was inserted into T cells, it created a unique new cell product named ‘IMPAC-T’ cells by the researchers, which effectively guided the T cells to kill cancer cells in laboratory experiments.
“It was incredibly exciting to take these minibinders, which were created entirely on a computer, and see them work so effectively in the laboratory,” says a co-author of the study.
The researchers also applied the pipeline to design binders for a cancer target identified in a metastatic melanoma patient, successfully generating binders for this target as well. This documented that the method also can be used for tailored immunotherapy against novel cancer targets.
A crucial step in the researchers’ innovation was the development of a ‘virtual safety check’. The team used AI to screen their designed minibinders and assess them in relation to pMHC molecules found on healthy cells. This method enabled them to filter out minibinders that could cause dangerous side effects before any experiments were carried out.
Precision cancer treatment on a larger scale is moving closer after researchers have developed an AI platform that can tailor protein components and arm the patient’s immune cells to fight cancer. The new method, published in the scientific journal Science, demonstrates for the first time, that it is possible to design proteins in the computer for redirecting immune cells to target cancer cells through pMHC molecules.
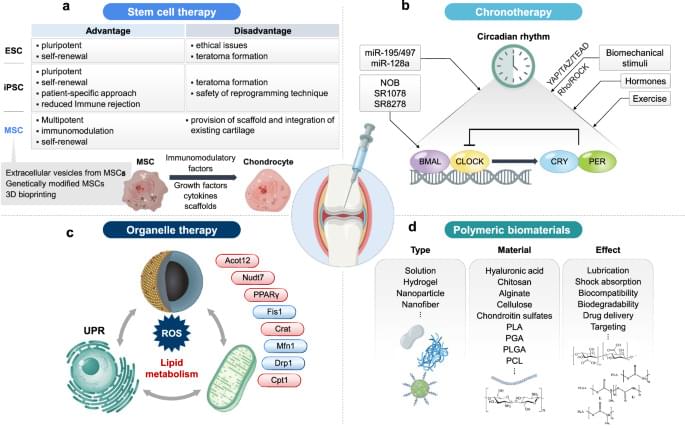
Osteoarthritis (OA) is a common joint disease that causes pain and stiffness, especially in older adults. Researchers are exploring new therapies to address this issue, here focusing on regenerative medicine, which uses stem cells to repair damaged cartilage. This involves injecting stem cells into joints to promote healing. However, challenges such as cell survival and long-term effectiveness remain. This study also examines gene therapy, which targets specific genes to reduce inflammation and cartilage breakdown. Biomaterials such as hydrogels and nanoparticles are used to deliver these therapies directly to the joint, improving treatment precision. In addition, this research highlights the role of circadian rhythms in OA, suggesting that timing treatments could enhance their effectiveness. These advancements aim to provide more personalized and effective OA treatments. Future research will focus on refining these approaches for better patient outcomes.
This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
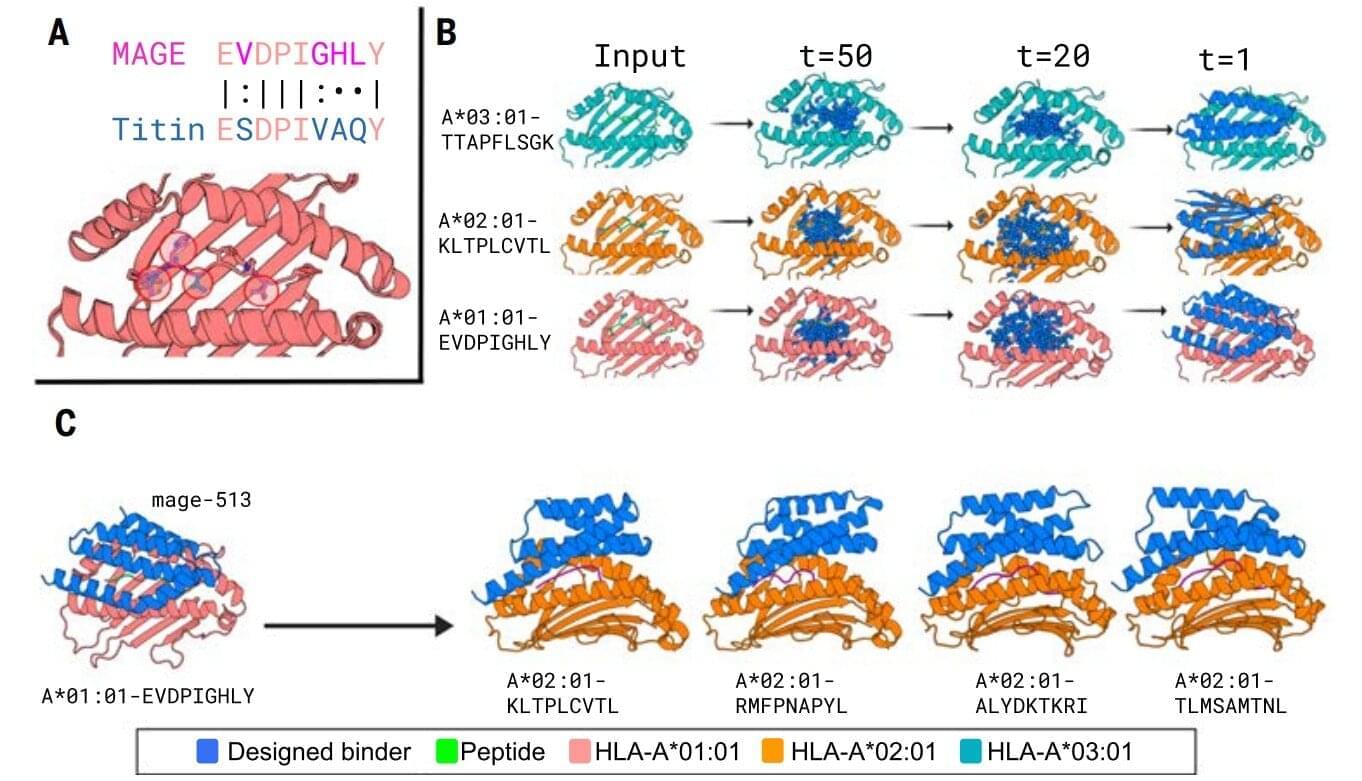
New designer proteins created using an AI tool can selectively target peptide segments that bind to markers on diseased cancer cells, acting like molecular flags that signal immune cells to attack and destroy the threats.
In a recent breakthrough, a team of researchers from the U.S. presented protein binders that specifically recognized the peptide portion of 11 diverse pMHCI complexes—amino-acid fragments found on the surface of almost all cells in the body that play a central role in the immune system’s ability to recognize and respond to abnormal or diseased cells, such as cancer cells.
These proteins, designed with the aid of AI, help human immune cells identify the correct targets and function more effectively, according to findings published in Science.
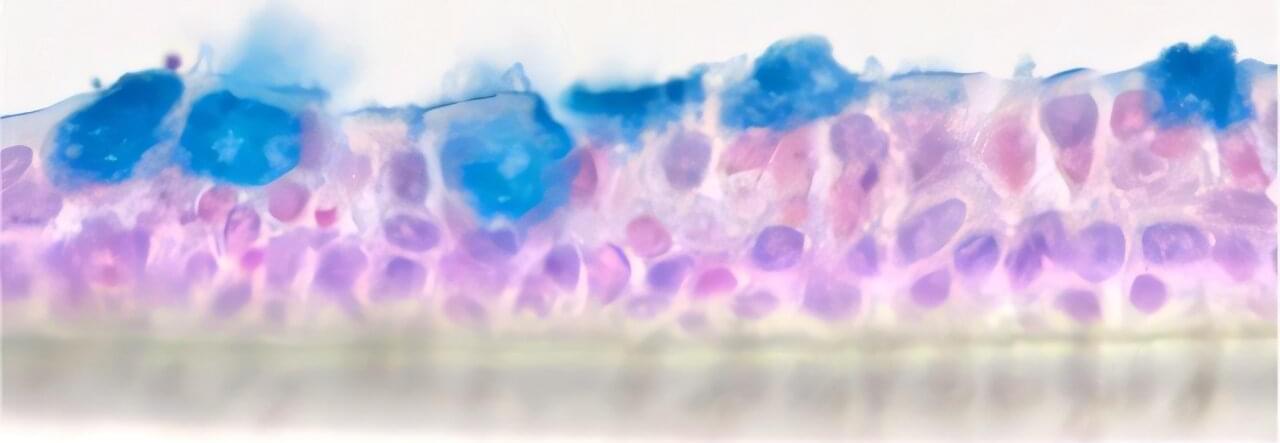
Patients with bronchial asthma suffer from attacks of shortness of breath caused by constricted airways. “Anti-inflammatory medications are usually given to treat this, although it isn’t quite clear how inflammation and constriction correlate,” says Professor Daniela Wenzel, head of the Department of Systems Physiology in the Faculty of Medicine at Ruhr University Bochum.
“These medications often stop working at a certain point.” Furthermore, asthma patients often experience a thickening of the bronchial tissue due to the accumulation of collagen. Goblet cells also form in increasing numbers, producing mucus and making breathing even more difficult. Currently, there is no medication to counteract these changes.